
Over the past 10 years, EWG has investigated the safety and efficacy of sunscreens made in the U.S. We have found that, due to the weak federal rules set by the Food and Drug Administration, many inferior products reach store shelves, touting overstated sun protection claims. EWG provides guidance to consumers on the significance of sunscreen as part of a sun protection strategy and highlights the sunscreen products that provide the best UV protection with the safest ingredients.
In this paper we review the health risks of exposure to ultraviolet rays, and the ability of sunscreens to protect people from UV-related skin damage. Most of the sunscreens on the market can prevent sunburn when used appropriately, and preventing sunburn is critical to lowering risks of squamous cell carcinoma. Sunburns, particularly those occurring in childhood, also increase a person’s risk of developing melanoma, the deadliest form of skin cancer.
However, despite record growth of the sunscreen market, American melanoma diagnoses are on the rise. Explanations for this trend are elusive. One factor could be the inferior UVA protection provided by American sunscreens. UVA rays, common with tanning beds, are known to increase melanoma risk. One study found that beachgoers using a poor quality “broad spectrum” sunscreen for two days on a tropical beach get the same dose of UVA exposure as those visiting a tanning salon once.
Another factor that could be increasing melanoma rates is that many people rely exclusively on sunscreen for UV protection, rather than using sunscreen in conjunction with protective clothing and other sun protection measures. When they use sunscreen only, they get more UV exposure.
Below we will discuss what is needed to formulate an “ideal” sunscreen and reasons why U.S. products fall short.
Section 1: How well does sunscreen prevent UV-related skin damages?We discuss the different wavelengths of UV rays and their roles in skin damage. Sunscreens are marketed by sunscreen protection factor, or SPF, a value that represents the degree of sunburn protection they offer but not how well they protect from UVA rays, which produce subtler skin damage.
Section 2: UV protection is key, but how do EWG and others measure it?There are four key techniques used to measure the degree of protection a sunscreen offers from UV rays. Sunburn protection ratings are based on tests using human volunteers and are not always repeatable from laboratory to laboratory. In vitro laboratory techniques can measure the amount of light filtered by sunscreen across the UV spectrum. They are better than human tests for quantifying the amount of UV shielded in the UVA range. To estimate UV protection, EWG uses a third method – modeling – which is based on the concentration of active ingredients on a product label. Modeling generally correlates well with laboratory measurements and allows us to look broadly at sunscreen performance across the U.S. market. The best indicator of sunscreen protection is measuring a sunscreen’s ability to reduce biological effects like DNA damage, immune system suppression and free radical generation – all of which are precursors to skin cancer. But these studies are expensive and rare..
Section 3: Do sunscreens live up to their claims?We reviewed the results of studies attempting to verify the performance of U.S. products. This data identified problems with unreliable SPF values on product labels, particularly for some mineral products. They also suggest some manufacturers are using inactive ingredients to boost SPF values and that many products lack strong UVA protection.
Section 4: What no testing measures – the real-world performance of sunscreenSunscreen protection is never as good in the real world as lab measurements suggest. There are several reasons why these products may not work as anticipated. SPF values are determined using an unrealistically thick coating of sunscreen – most people don’t apply the recommended amount and fail to reapply as directed. Meanwhile, some forms of sunscreen don’t form a thick and stable coating on people’s skin, and others separate or clump over time.
Section 5: What must be done to improve the UV protection of sunscreens?The FDA should tighten sunscreen rules to improve the performance and marketing of U.S. products. Chemical manufacturers must work with the FDA to get new, UVA-filtering active ingredients on the market. In the interim, sunscreen manufacturers must work around these limitations to make stable products that offer a better balance between UVA and UVB protection. Consumers must recognize limitations of current sunscreens, select better products and use them in conjunction with other methods of UV-exposure protection.
In 1978, when the FDA initiated its rulemaking on sunscreen, the agency stated that all deleterious effects from the sun were caused by the UVB rays that led to sunburn, writing in the Federal Registry:
The Panel is particularly concerned about recurrent sunburn and overexposure to the sun throughout the years, because the lower wavelength limit of cancer-producing radiation on the skin of mice and rats has been shown to be 325 [nm] , i.e., the same spectral range that produces sunburn in human skin.1
But in the past 30 years, scientists have determined that lower-energy UVA rays in the 325 to 400 nm range also cause photodermatoses, immune system suppression, photoaging and cancer.2,3,4,5
People typically buy a sunscreen based on its sun protection factor, or SPF, thinking higher numbers mean better sun protection.6 The SPF value is a measure of how well a product shields the skin from high-energy UVB rays. In addition to causing sunburn, UVB rays can damage DNA in skin cells, contributing to skin cancers.
But the SPF value is not an indicator of protection from all UV-related skin damages. Although many U.S.-made sunscreen products claim “broad spectrum” sun protection, which implies protection from both UVB and UVA rays, they actually vary substantially in the degree of UVA protection they offer. Since the FDA initiated its rulemaking for sunscreens in 1978, clear evidence has emerged that lower-energy UVA rays are more numerous, penetrate deeper into the skin and can promote sun tanning without causing sunburn. Solar rays in the UVA wavelength form free radicals, which are highly reactive, excited, unpaired electrons that damage skin tissue. Exposure to UVA radiation activates inflammatory cytokines and inhibits the skin’s immune response, possibly setting into motion another mechanism that facilitates the development of skin cancer. Tanning beds primarily emit UVA radiation to promote tanning and have been directly linked to increased rates of melanoma.7
The process of manufacturing a sunscreen that filters UVA rays is more challenging than making a sunscreen that prevents sunburn. As a result, sunscreens don’t shield skin from UVA rays as efficiently as they do UVB rays. For example, although products with SPF values above 15 reduce UVB by 93 percent or more, studies show that sunscreens generally reduce free radical generation by 50 to 75 percent.9,10 The immune system protection of a sunscreen is more likely to correlate with its amount of UVA-shielding than its SPF.11 Therefore, an inferior sunscreen can successfully prevent sunburn without protecting the body from UV-related immune system suppression and skin cancer.
Graphic 1 – Sunburn is primarily caused by UVB rays, but UVA rays cause tanning, skin-damaging free radicals and immune system suppression.
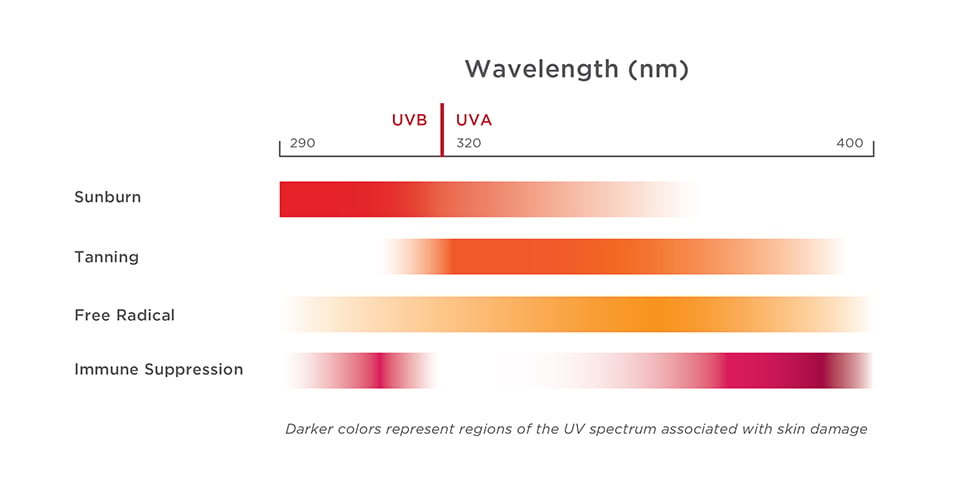 Source: Osterwalder, et al., 20098
Source: Osterwalder, et al., 20098
The FDA allows most sunscreens to claim they contribute to preventing skin cancer, but it simultaneously reports that the evidence to support this statement is contradictory.12 The available evidence suggests that regular sunscreen use prevents squamous cell carcinoma. Scientists have not presented strong evidence that sunscreen use prevents basal cell carcinoma – in part because regular sunscreen users may spend more time outside and get more sunburns and UV exposure than people who do not use sunscreen or who cover up or spend less time outdoors.
The biggest question about sunscreen today is whether it can help prevent melanoma, the deadliest form of skin cancer. For decades, the rate of melanoma diagnoses has grown faster than that of any other kind of cancer, with no satisfying explanation for the rapid increase. In 1978, when the FDA initiated its sunscreen rulemaking, the National Cancer Institute reported that about 9,000 Americans had been diagnosed with melanoma. This year, the institute estimated that more than 91,000 people will be diagnosed with melanoma.13
UV exposure has a complicated connection to melanoma risk. Both UVB and UVA rays appear to contribute to it. Early life sunburns increase lifetime risk, as does the use of tanning beds, which subject people to strong doses of UVA rays. Only one prospective study supports the notion that sunscreen use prevents melanoma. Set in Australia, this study found that daily use of an SPF 15 sunscreen, in addition to other sun protection strategies, reduced the number of new melanoma cases by 50 percent and of invasive melanoma by 71 percent.14
Usually, increasing exposure to a hazard – whether a toxic chemical or UV radiation – leads to heightened risk. But this may not be the case for UV radiation and melanoma. Instead, some studies indicate that regular sun exposure is associated with lower melanoma risk,15 as shown by its lower incidence in outdoor workers and people living in the American Sunbelt, compared to those living in cooler, more northern latitudes.13
Because of complexities involved in how UV light causes harm, scientists have not developed a universally accepted way to measure a sunscreen’s ability to protect people from melanoma or the long-term skin damage caused primarily by UVA rays. The interim conclusion is that sunscreens should evenly filter light through the UVB spectrum, as well as the shorter and longer wavelengths in the UVA range, known as UVAI and UVAII, respectively.16
Because the origins of melanoma are imperfectly understood, as is the role of UVA in other forms of sun-related skin damage, scientists have advocated for sunscreens to provide “uniform protection” that strives to shield skin evenly through the entire UV spectrum. Fabric is a model for ideal sun protection. A basic cotton weave provides a skin protection factor of at least five across the UV spectrum. Although this number is much lower than the SPF advertised on most sunscreen bottles, sunscreen breaks down and washes off. In contrast, clothing offers ample protection from sunburn and the best possible balance between UVB and UVA rays.16
The FDA regulates sunscreen as an over-the-counter drug. Sunscreen manufacturers are limited to using 17 FDA-approved ingredients in sunscreen,12 but only eight of these chemicals are commonly used. Most approved ingredients filter only a portion of the UV spectrum. Sunscreen formulators typically mix one to six ingredients to create products that offer varying degrees of protection across the UV spectrum.
Although many of the available ingredients filter UVB rays effectively, fewer cover the UVA spectrum. Only zinc oxide and avobenzone protect against shorter and longer UVA rays. To achieve high-SPF values, American sunscreen manufacturers must add UVB filters, which drive up the SPF value but result in a poor balance between UVA and UVB protection.
Graphic 2 – Only two of the UV filters allowed in the United States provide meaningful UVA filtering.
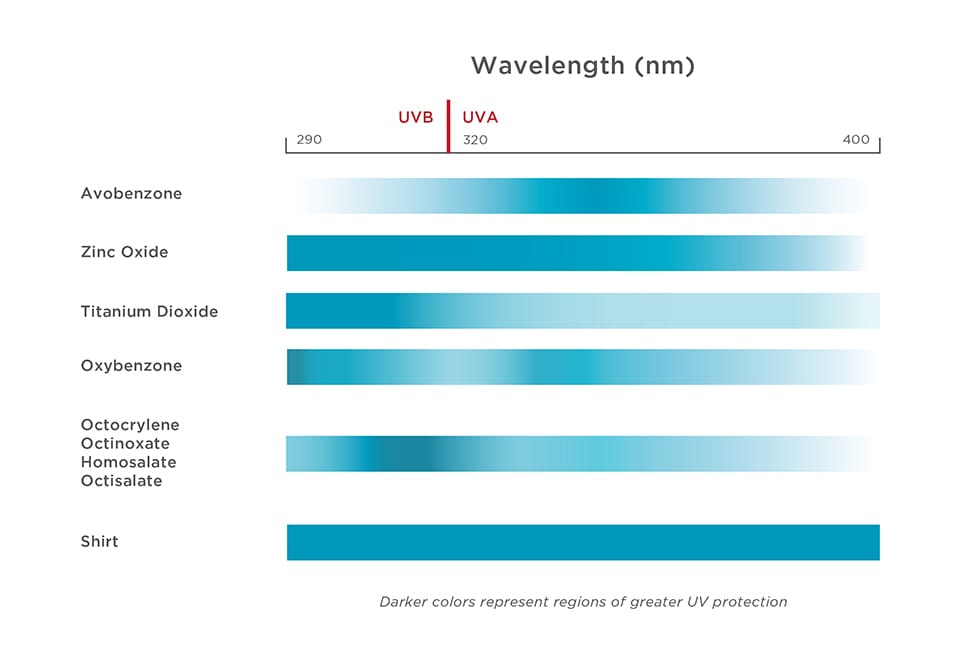
Many American sunscreens effectively shield users from sunburn but still allow too many UVA rays through. Without the painful reminder of a sunburn, people have no indication that they have received harmful doses of UV rays, and they may use sunscreens to rationalize prolonging their time in intense sunlight.17
Most other industrialized nations require a stronger proportion of UVA protection in sunscreens and cap SPF values at 50+, a practice that ensures a better balance between UVB and UVA shielding. European sunscreen guidelines require that a product’s UVA protection be at least one-third as strong as its UVB protection. The FDA considered a similar system for rating UVA but instead, in 2011, set weak UVA protection rules that enable nearly every product to achieve a passing grade without reformulation and then permitted those products to advertise “broad spectrum” protection.
The FDA’s restriction on allowed active ingredients and combinations is an important factor limiting American manufacturers from making products that are more protective. The agency has notified sunscreen manufacturers that it will not approve new, modern ingredients that offer stronger, stable UVA protection – specifically, four ingredients widely used in Europe could provide a much-needed boost to the UVA protection of American-made products – until the ingredient makers provide more information about safety tests.18
This is not just a theoretical concern. British researchers Brian Diffey and Uli Osterwalder of BASF assessed the potential impacts of using a poor-quality sunscreen, estimating that over a two-week vacation in a tropical latitude, a fair-skinned tourist could successfully prevent sunburn but receive as much UVA exposure as he or she would by visiting a tanning salon 10 times for eight-minute sessions.19 At this rate of overexposure to UVA radiation, just three days in the sun using sunscreen would be equivalent to two trips to the tanning salon. Even one visit to a tanning salon increases the risk of developing melanoma and other skin cancers.20 All of the sunscreens assessed in the study met the FDA standard for “broad spectrum” protection and could legally claim to help reduce risk of skin cancer in the U.S.
Given the limitations of sunscreens, it is best to consider these products just one element of a sun protection strategy that prioritizes shade, protective clothing and hats, and outdoor activities planned for less sun-exposed times of day. Moreover, EWG strongly encourages individuals to schedule routine dermatology exams. In 2014, EWG launched its Sun Safety Campaign, in conjunction with several sunscreen manufacturers, to spread the word about safer sun practices.
Graphic 3 – U.S. sunscreens offer less UVA filtering than European sunscreens or clothing.
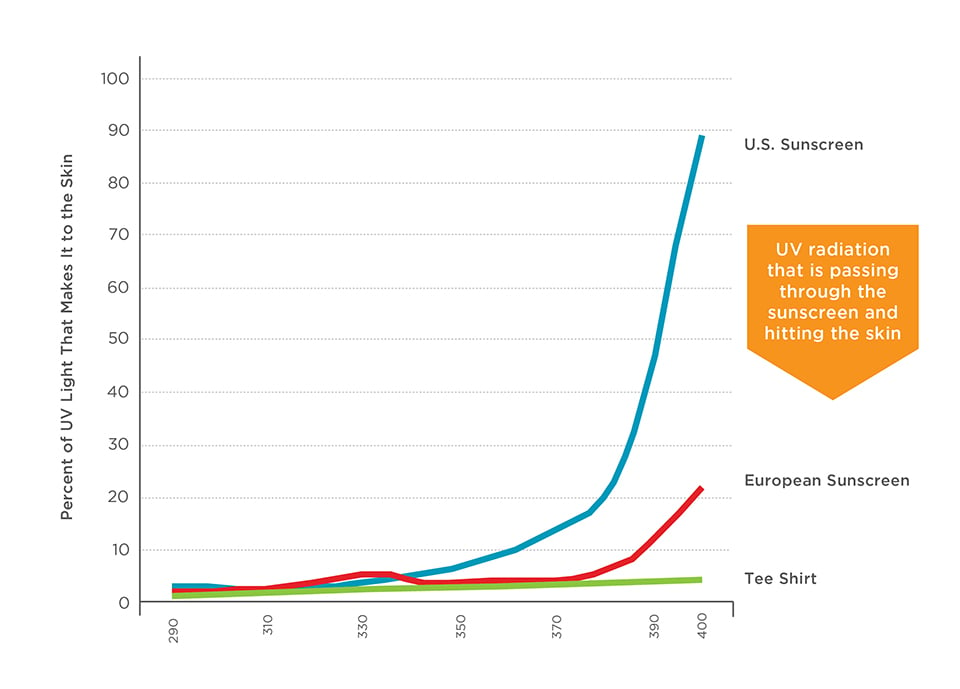
Source: Diffey, 201519
Despite growing awareness of what makes a good sunscreen, scientists’ opinions differ on how to measure and grade UV protection. Researchers use four common methods to evaluate sunscreen effectiveness. Scientists can observe human volunteers in controlled tests of SPF and persistent darkening of skin pigment. Alternatively, product efficacy can be assessed by smearing sunscreen on a glass or acrylic slide and using a spectrophotometer to measure the amount light passing through it. UV protection can be estimated using mathematical models that take into account the amounts and combinations of active ingredients. Uncommon but potentially very important methods include measuring a sunscreen’s ability to prevent other UV-related skin damages. Each of these approaches has benefits and drawbacks.
In-vivo testing – The FDA requires all sunscreens sold in the U.S. to be tested by exposing 10 volunteers to a high-intensity UV lamp.12 The next day, investigators examine their skin for reddening. The agency allows manufacturers to discard results from three of the 10 test subjects and base the labeled SPF value on the amount of UV that caused a sunburn in the remaining seven participants. This approach leads to greater SPF values on product labels. Tests on human volunteers are expensive, and some argue it is unethical to expose people to damaging amounts of UV light.
The benefit of SPF testing is that it measures the direct response of the human body. One major drawback is that this type of test cannot differentiate between good and poor UVA protection. In addition, the results of SPF tests can vary widely from one lab to another. European researchers tested 44 European sunscreens for sunburn protection and found that in many instances, their results did not match what was on the label. High SPF products were the most likely to suffer from poor repeatability. They concluded that products sold to consumers as SPF 50 could have test values of SPF 30, 50 or 100 in their experiment.21
A similar method for evaluating UVA protection in humans is the immediate pigment darkening, or IPD, test, which exposes volunteers to shorter UVAII rays and examines how much tanning results. The IPD test is measured directly in humans. However, the IPD value doesn’t reflect the amount of protection a sunscreen provides from lower-energy UVAI rays. This method would presumably show similar variations among labs that are similar to the in vivo SPF tests. The FDA does not require IPD testing. Judging from the lack of available data, this method does not seem to be widely used, but some sunscreen manufacturers indicate they conduct IPD tests on their products.
In-vitro testing – UV shielding can be measured in a laboratory using spectrophotometry. Scientists spread a layer of sunscreen on a quartz or acrylic slide and measure exactly how much UV is deflected by the sunscreen and how much passes through the slide. In vitro tests are less expensive than other test methods and have better repeatability. Unlike tanning, they can quantify UVA protection in the UVAI spectrum. The results can be summed up in various ways to estimate the degree of UVA shielding and convert it to a UVA protection factor. It is easier to calibrate results because of the controlled lab setting, but the type of UV lamp used, the composition of the slide – quartz or acrylic – and the surface roughness of the sunscreen on the slide can all affect the measurements.
In-silico testing – EWG models UV protection based on the active ingredients listed on each product label and inactive ingredients that may stabilize the formulation. Using Beer-Lambert’s law, we calculate how much light will pass through a sunscreen and the expected UVB and UVA protection. International test data have shown that models such as ours are generally good at predicting the efficacy of both the slide-based methods and human subject test methods of evaluating sunscreens.
This approach to estimating SPF was first published in 1979 by Richard Sayre, then an employee of Schering-Plough.22 This is the method used now and made readily available to sunscreen formulators by BASF, the world’s largest chemical producer and one of the largest producers of active ingredients for sunscreens. BASF researchers published an updated methodology document in 201523 and made a free interactive tool available online.24 The BASF sunscreen simulator has been shown to have “very good correlation between SPF in-silico and SPF in-vivo” and to make “realistic estimations of the final product performance.”25
The EWG methodology for our sunscreen guide follows the approach used by BASF and Sayer. It uses the estimated UV absorbance for each of the active ingredients allowed in U.S. products.26 EWG has made minor adjustments to the methodology for two mineral sunscreen ingredients – titanium dioxide and zinc oxide – to match the types of those ingredients most commonly used in U.S. sunscreens.
The benefit of EWG’s method of UV modeling is that it has been validated against other laboratory tests, including in vitro tests we have commissioned. This method allows us to examine and rate the UV protection of more than 1,000 products on the market. One drawback is that, as with in vitro testing, we cannot evaluate the role of inactive ingredients that may mask reddening or boost UV filtering. This factor is captured only in human tests. Our modeling also cannot identify problems that may be caused when a sunscreen is poorly formulated and ingredients clump or separate instead of being distributed evenly throughout the mixture before application. Both spectrophotometry and tests on humans can identify these problems.
None of the methods described above accounts for the real-world use of a product in the sun or ease of use, which is affected by the thickness, stickiness and aesthetics of a product. Nor do they evaluate the performance of a product that has sat on a shelf or in a hot car for months.
Measuring the degree of protection from skin damage – The most accurate method of gauging sunscreen protection is measuring the degree to which a product reduces biological damage caused by UV rays, instead of the outward indicators of skin tanning or sunburn. This method is rarely used with real-world products. In scientific studies, researchers use various methods to measure the degree of protection for UV-initiated free radicals,9,10,27 measure immune system reaction to a known allergen to estimate immune protection factors of sunscreens,11,28,29 or measure the reduction in UV-initiated DNA damages.30
The available data on this aspect of sunscreen performance is outdated by several years or allowed only in other countries. Yet, collectively, the data suggests that sunscreens are not as effective at preventing free radical formation, immune system suppression and DNA damage as they are at preventing tanning and sunburn.
The FDA and dermatologists should continue to investigate the efficacy of today’s sunscreens. Companies should formulate sunscreens that provide the highest degree of protection from the full range of UV-related damages. Marketing sunscreens based on their ability to prevent sunburn only misleads consumers about the protection they may or may not receive from UVA rays and sun damage.
EWG and other researchers have reported serious differences between some sunscreens’ advertised UV protection and the results of laboratory tests. SPF values on labels are not always confirmed by independent tests in human volunteers, spectrophotometry or modeling. U.S. products claiming “broad spectrum protection” may not be sold in other countries where the requirements for UVA protection are stronger. Deficiencies are notable in products with ultra-high SPF values. These data suggest that the FDA should investigate discrepancies in sun protection and set stronger rules for sunscreen.
SPF and sunburn protectionThe FDA requires sunscreen manufacturers to set product SPF values based on human tests, using a minimum of 10 human volunteers. The agency requires manufacturers to verify products’ water resistance claims by testing sunburn after volunteers immerse themselves in a pool of water for 40 or 80 minutes. Yet SPF values reported on product labels do not always match those predicted by other types of tests. In general, confirmation lab tests suggest that many sunscreens offer far less sunburn protection than is advertised on labels. Minor variations in test conditions lead to big differences in SPF values. When products are tested outdoors in natural sunshine, the SPF is lower still.31
Consumer Reports tests sunscreens on human volunteers to confirm whether the SPF values are accurate. It uses a slightly different method than the FDA mandates for sunscreen companies, and it does not disclose the number of human test subjects used for each sunscreen. Yet it chronically finds problems with sunscreens’ achieving far lower SPF values in its tests than what is claimed on their labels.
In 2015, Consumer Reports tests found notable variations in SPF values for mineral-only sunscreens, products that mixed minerals with non-mineral filters, and products advertising SPF values greater than 50+.32 In 2016, it reported similar shortcomings in SPF protection, with some notable issues for some mineral sunscreens and high-SPF products. It reported that non-mineral sunscreens had, on average, 86 percent of the labeled SPF value, and the SPF for mineral sunscreens was on average 64 percent of the labeled value33 (see Table 1 below). The sample sizes were relatively small and non-random, with some overlap between 2015 and 2016. Although the methods used to test sunburn protection differed slightly, the reasons for the discrepancy between Consumer Reports and manufacturer’s reported SPF values are unclear.
Table 1: Consumer Reports 2016 test results – comparing in vivo SPF testing with labeled SPF values| N= | Meeting SPF Claim (75% or Greater of Labeled Value) |
Average Consumer Reports SPF vs. Label Value |
|
|---|---|---|---|
| Mineral sunscreens | 18 | 39% | 64% |
| Non-mineral sunscreens | 51 | 69% | 86% |
Consumer Reports found that mineral products with similar concentrations of active ingredients found notably different SPF values in its tests. For example, the 2016 report examined seven mineral sunscreen products with relatively low concentrations of both zinc oxide and titanium dioxide (ranging from 3.7 percent to 7 percent of each mineral). Package labels reported SPF values of 30 to 50+. Two of seven products met the labeled SPF value, whereas the remaining three offered only 16 percent to 50 percent of the advertised SPF.
The Consumer Reports investigation highlights problems with the FDA test methods for SPF values. SPF tests conducted by manufacturers should match results of those conducted by another lab. Replications should produce consistent results for the same product. Where there is person-to-person variation, the lowest reported SPF values should be listed on product labels rather than discarded as outliers, as is currently allowed in FDA tests.
Ultra-high SPF claimsEWG is concerned about the continued sale of ultra-high-SPF sunscreens. The FDA proposed capping SPF values at 50+ in 2011, calling ultra-high-SPF values “inherently misleading.”34 SPF values are capped at 50+ in Europe, Australia, Canada and Japan.35 There is little evidence to indicate that higher SPF products provide any additional health benefits. To the contrary, some studies suggest that high SPF products may mislead consumers into believing that they are fully protected from sun damage. One result is that people using sunscreens with higher SPF values risk excessive exposure by spending more time in the sun.17
The main argument in support of high-SPF products is that since people do not adequately apply and reapply sunscreen, higher-SPF products may compensate for inadequate application.3637,38 Another factor behind the FDA’s proposal to cap SPF values at 50+ is that human test methods cannot reliably measure differences between ultra-high SPF values. The FDA’s method tests sunburn on small areas of a volunteer’s skin. Each site is treated with just one milligram of sunscreen. Tiny differences in the amount of sunscreen used or application thickness could result in major differences in the calculated SPF. These errors would be most dramatic for high-SPF products.
Sunscreen giant Procter & Gamble supports the FDA-proposed cap, arguing that a very small difference in test conditions can have a dramatic influence on the calculated SPF. In a letter to the FDA, P&G calculated that a 1.7 percent change in the UV transmission yields a SPF measurement of 37 instead of 100.38 It also warned that the intense UV light used in lab SPF tests was different from conditions experienced in the real world and of “dubious value.” Johnson & Johnson scientists disputed this assertion in a paper, reporting that multiple labs confirmed SPF values of 70 and 90 in products tested on human volunteers.39
In addition to questions about consumer perceptions and test methods, it is not clear how manufacturers are formulating products with these sky-high SPF values. Values greater than 70 do not appear to be feasible, given the active ingredients currently approved by the FDA. But EWG’s model predicts lower SPF values for most products that claim SPF values greater than 40, with a bigger discrepancy for SPF values above 50+ (see Table 2 below).
Table 2: EWG estimated SPF versus labeled value| Label SPF | N= | Average EWG estimated SPF vs. labeled SPF |
|---|---|---|
| 30-39 | 339 | 104% |
| 40-50+ | 263 | 82% |
| >50+ | 86 | 65% |
In vitro testing also suggests shortcomings in sunburn protection for high-SPF products. In 2009, Jay Nash of Procter & Gamble tested 330 U.S. sunscreens and submitted this data to the FDA to assess the potential effects of the sunscreen rules proposed two years earlier.40 Nash measured the amount of UV radiation that passed through a layer of sunscreen spread on acrylic slides and found that high-SPF products typically offered less UVB shielding than is touted on their labels. As with the EWG model, products with the highest labeled SPF values performed the worst. In Nash’s tests, products with SPF values of 70 and higher exhibited the greatest differences between labeled and estimated SPF. The measured SPF was less than half of the labeled value for 12 of the 14 products he tested.
These findings suggest that manufacturers may be adding ingredients to boost the SPF values on labels. These ingredients do not change the UV-transmission through a slide in the laboratory, which implies that they do not prevent UV radiation from striking the skin. Rather, they would use other mechanisms to reduce the presence of sunburn once UV rays strike skin. The FDA should investigate the use of inactive SPF boosters, their performance, stability and ability to protect skin from other types of UV-related skin damages.41
UVA or ‘broad spectrum’ protectionBy all accounts, UVA protection remains a challenge for U.S.-made sunscreens. EWG analysis suggests that many products bearing the “broad spectrum” label could not be sold in Europe, where UVA protection must be at least one-third as strong as the labeled SPF value of the product. According to our modeling, only 3 percent of beach and sport sunscreens would fail the FDA critical wavelength test for broad spectrum protection. Yet we estimate that 49 percent of the more than 750 beach and sport sunscreens in our 2016 database pass the FDA broad spectrum test but would not pass the European UVA test.
Our findings are in line with Procter & Gamble’s 2009 product survey, which reported that 68 percent of sunscreen products manufactured for that year would pass the FDA’s Critical Wavelength test for broad spectrum protection. The company estimated that only 44 percent could be sold in Europe.40 Without a system that rewards superior performance, American manufacturers have little motivation to improve the UVA shielding of their products.
Higher-SPF products provide less balanced UVA protection in relation to UVB protection. Analyzing data submitted by Procter & Gamble, EWG determined that the average UVA Protection Factor was 50 percent of the measured SPF for products with labeled SPF values between 30 and 50 but decreased to about 33 percent for products with labels for SPF over 60.
This decrease in UVA protection relative to that of UVB is in part due to FDA rules limiting the use of ingredients that filter UVA rays. For example, avobenzone and zinc oxide are the only allowed ingredients that provide good UVA shielding. Nearly every non-mineral product with SPF of 30 or higher uses avobenzone at the maximum concentration of 3 percent. As the SPF in these products increases, the UVA protection does not increase proportionally.
Recently, British researcher Brian Diffey evaluated four SPF 50 and 50+ sunscreens sold in the U.S. and four sold in Europe. The European products featured modern UVA filters not allowed in the U.S. He found that the U.S. sunscreens allowed an average of three times the UVA rays to pass through to skin, compared to those sold in Europe.42 We would expect the UVA balance to be even worse for U.S. sunscreens with SPF values greater than 50+.
Yet American sunscreen manufacturers may not be able to improve UVA protection until the FDA caps SPF values and approves the new, modern filters used in Europe. In vitro tests by Steven Q. Wang of Memorial Sloan Kettering Cancer Center found that only products with Tinosorb and Mexoryl SX achieved the highest rating on a proposed “spectral uniformity” test.43
Graphic 4 – High-SPF European sunscreens block more UVA radiation than U.S. products. Tests of eight SPF 50 or 50+ sunscreens from the U.S. and Europe.
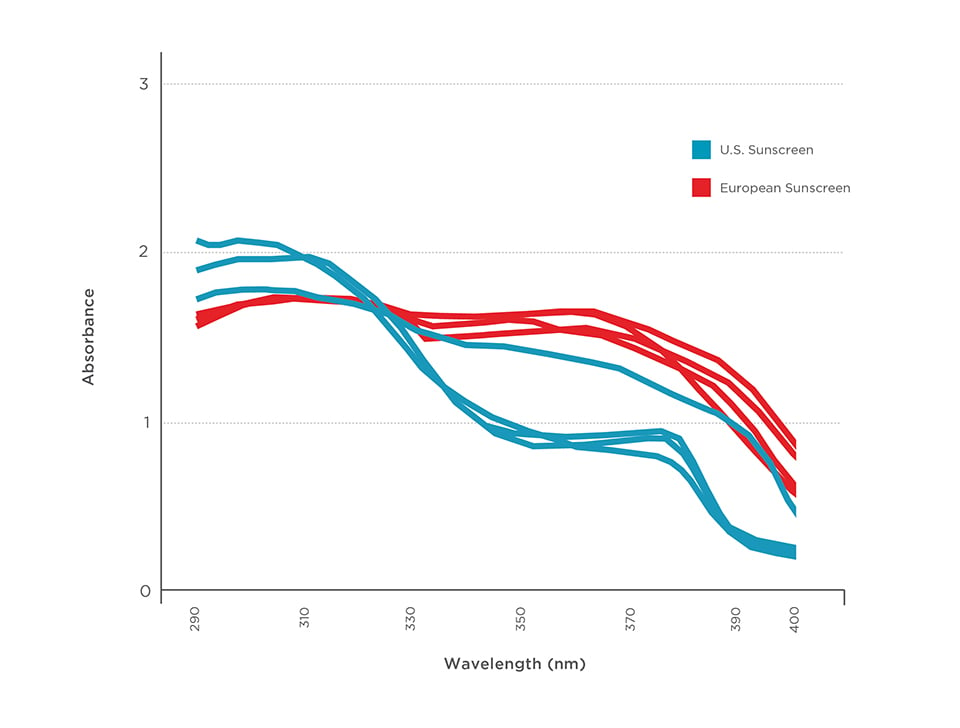
Source: Diffey, 201543
A wealth of evidence suggests that today’s sunscreens drastically overstate the skin protection they offer from UV damages.
SPF values on sunscreen labels are similar to the gas-mileage estimates on new cars: They represent ideal performance under unrealistic test conditions and often misrepresent outcomes in the real world. Real-world UVA shielding and balance are harder to gauge, and subtler skin damages may show up only decades later.
This problem can result from a variety of factors:
A) SPF is tested using an unrealistically thick coat of sunscreen, far more than people use in the real world.The test requirements for sunscreen specify application of an unrealistically thick coating of sunscreen to the skin surface or test slide. This would be the equivalent of a family of four using a four-ounce bottle of sunscreen for a two-hour visit to the beach. In reality, people apply far less sunscreen to their skin and do not reapply it often enough to achieve the advertised SPF. UV protection does not follow a linear relationship, which means that if you apply half of the recommended amount, you get less than half of the labeled SPF protection. Underapplying sunscreen results in far less sunburn protection and UVA protection. A thinner coating of sunscreen diminishes UVA protection slightly more than UVB protection.44
SPF tests by companies evaluates a similarly thick coat of sunscreen on all volunteers, but in reality, people apply products differently, on the basis of packaging and skin feel. Thicker pastes, such as stick sunscreens and mineral formulations, feel heavier on the skin. It’s easy to believe a thicker coating has been applied than is the case. Water-oil emulsions form the most uniform layer on skin. Aerosol sprays are dispensed in tiny droplets that form the thinnest film on skin, with more gaps.45 EWG is concerned that these qualities make it easier for sunscreen users to miss some areas during application. In 2011, the FDA asked manufacturers to submit data proving that aerosol sprays provided a thick enough coating to impart good skin protection.12
A recent industry-funded study of 52 consumers found that people using sunscreen lotions applied an average of 1.1 mg/cm2 to their forearms, whereas those using sunscreen sticks applied far less, at 0.35 mg/cm2.46 Sprays were tested using various methods. Volunteers were asked to spray a “normal” amount onto a diagram of a person, and the quantity of sunscreen applied was measured by weight. Researchers reported that the volunteers using aerosol sprays applied average spray thickness of 1.6 mg/cm2. EWG cautions that testing with a diagram instead of a person probably leads to an overestimate of the real-world sunscreen spray application.
The same study reviewed other sunscreen application research, which reported a range of application rates, from 0.11 to 1.48 mg/cm2 – all far less than the 2.0 mg/cm2 specified in SPF tests.46 One study found that people applied mineral sunscreens in thinner coats than chemical sunscreens, a pattern they attributed to the denser, thicker nature of mineral products.47
These studies raise a fundamental question: Why not label SPF values according to real-world application behaviors and communicate a more accurate message about sunburn protection?
B) Sunscreens commonly include ingredients that affect skin reddening and UV protection but are not listed on product labels as “active ingredients.”Tests of real-world sunscreen along with EWG modeling suggest that sunscreen manufacturers commonly add unregulated ingredients to sunscreens – ingredients that can have a major effect on SPF values. Many of them may decrease skin reddening, but it is not clear whether they also reduce other types of UV damage. SPF boosters limit skin redness in human studies but do not necessarily block UV light from striking the skin as measured by spectrophotometry or in EWG modeling.
Ingredients that act as skin antioxidants and anti-inflammatories can reduce sunburn. Other ingredients, such as hollow spheres, can boost the UV protection of active ingredients by scattering UV rays.
UV scattering agents bend UV rays so they pass through a thicker layer of sunscreen and have more opportunity to be absorbed or scattered by active ingredients.48 These substances should provide a benefit similar to a thicker layer of sunscreen, and affect both UVA and UVB rays. Dow’s Sunspheres™ are hollow spheres approximately 250 nm in size made of styrene/acrylates co-polymer (i.e., plastic), which the company claims can boost UV protection by 60 to 70 percent.
Anti-inflammatories inhibit skin reddening. EWG is concerned that many mask the immediate signs of skin damage without offering greater skin protection from UV damage. French sunscreen researcher Céline Couteau wrote:
The continuing anti-inflammatory effect, without reapplying the product at all, gives the user a sense of false security on the one hand, and on the other hand is likely to encourage them to prolong time of solar exposure.49
In 2013, Robert Sayer and John Dowdy petitioned the FDA to ban five active ingredients from two families of UV filters, because they appeared to inhibit skin reddening but not necessarily impart protection from other UVB damage.50 The petition cites one active ingredient, trolamine salicylate, which is not widely used in sunscreen but is marketed in anesthetic creams. A much more common ingredient, oxybenzone, is used in approximately 40 percent of sunscreens in EWG’s 2016 database. Oxybenzone was originally patented for its capacity to reduce skin redness when applied after sun exposure.51 Other widely used active ingredients, including homosalate and octisalate, appear to offer similar anti-inflammatory effects.
Other ingredients common in sunscreens have anti-inflammatory effects but do not change the amount of UV rays that hit skin. These include common botanical extracts from licorice, chamomile and aloe, among others. Couteau found that the addition of bisabolol, a component of chamomile, glycyrrhizate from licorice, and allantoin had potent anti-inflammatory effects on skin in laboratory studies.52 The anti-inflammatory effect can persist for more than six hours following application.49 Couteau has also shown a lack of relationship between the SPF value and the sunscreen’s anti-inflammatory activity.49
Some researchers dispute these concerns about ingredients with anti-inflammatory effects.36,53 They cite studies showing that, in addition to inhibiting sunburn, high SPF sunscreens protect against biological markers of UV skin damage.30,54
Antioxidants such as vitamin A, C and E, and botanical extracts are common in sunscreen. They are added based on the theory that these substances may impart additional skin protection by “quenching” or reducing free radicals caused by UVA rays,55 which damage skin DNA, proteins and lipids. Some studies find that the addition of antioxidant vitamins and botanicals like caffeine and echinacea have reduced skin damage.56
However, other studies have found that antioxidants have few biological effects.27 One critical factor is the difficulty formulating a lotion with antioxidants that does not break down in storage or during exposure to sunlight.56 Although antioxidants can stabilize products’ active ingredients, like avobenzone, they can also be broken down by UV rays, sometimes forming harmful byproducts. For example, research indicates that vitamin A additives, which are common in sunscreens, may speed the development of tumors and lesions on sun-exposed skin.57
EWG has asked the FDA to examine the use of SPF boosters and other inactive ingredients in sunscreens.41
C) Poorly formulated or old sunscreens may separate, making it impossible to coat skin evenly.To work effectively, sunscreens must form a stable film on the surface of the skin. The more uniformly UV filters are dispersed on the skin’s surface, the better protection they confer. Formulation is more challenging in higher-SPF sunscreens, in which the concentration of active ingredients can approach 25 percent in the case of mineral products, and go up to 40 percent in the case of non-mineral products sold as lotions or sprays.45 Sunscreen formulators use a variety of ingredients, such polymers, to try to ensure the UV filters are dispersed in an even, stable film on the skin. They must balance a high concentration of active ingredients optimally dispersed on the skin with consumer preferences for a light, spreadable formula.
Water- and sweat-resistant formulations use polymers or other technologies to resist degradation. EWG’s UV modeling and in vitro sunscreen testing do not assess these qualities in sunscreen formulations. The FDA requires sunscreens marketed as water-resistant to submit third-party verification of this claim. The FDA does not require manufacturers to prove that aerosols marketed for use on wet skin provide the sun protection they claim. By contrast, Canada requires companies to submit data to justify claims that a product works on “wet” or “sweaty” skin.58
Over time, cycles of heating and cooling can cause sunscreen ingredients to separate or clump in the bottle or tube. These products will not provide a thick, uniform coating on the skin surface, and will lead to poor skin protection and sunburn. Some sunscreen labels instruct users to shake the product before applying. Others are stamped with expiration dates.
D) Some sunscreens contain active ingredients that break down in the presence of UV rays.Avobenzone, the primary UVA filter in non-mineral sunscreens, can degrade when exposed to solar rays. Manufacturers improve stability by mixing avobenzone with other active ingredients, such octocrylene or inactive ingredient stabilizers, to slow UV degradation, with varying degrees of success.59
EWG’s UV protection model rates the photostability of the combinations of active ingredients disclosed on product labels. However, EWG’s methodology does not assess the degree to which specific products break down in the presence of UV rays.
Mineral-only sunscreens are the most stable and should not lose their ability to filter UV rays when they are exposed to UV rays. In spite of that, some forms of titanium dioxide, and to a lesser extent zinc oxide, can be activated by UV rays and form free radicals that damage surrounding cells. Sunscreen manufacturers generally use minerals with an inert coating that reduces the potential for photoreactivity. These coatings must be stable in sunlight.
EWG’s review of information from mineral sunscreen suppliers suggests that the forms available for U.S. sunscreen are coated to reduce photoreactivity, but unlike European regulators, the FDA does not set specifications for mineral ingredients in its sunscreen rules.
Consumers face a market saturated with sunscreen products, each bearing various performance claims. EWG aims to educate people about shortcomings in sunscreen, push the FDA and the industry to improve their performance, and guide consumers to the best available choices.
EWG’s recommendations:
The FDA must:In the absence of strong rules and a responsive regulatory agency, EWG advises consumers to be smart about sun safety. Although sunscreens are an important tool for preventing sunburn, and shoppers can use EWG’s Guide to Sunscreens to find better products, sunscreens should not be a person’s only tool to prevent sunburn, nor should they be used to prolong time spent in the sun. Protective clothing, sunglasses, hats and sun avoidance are more reliable ways to protect the skin from UV rays.