
Summary. Independent laboratory tests found a toxic food-can lining ingredient associated with birth defects of the male and female reproductive systems in over half of 97 cans of name-brand fruit, vegetables, soda, and other commonly eaten canned goods. The study was spearheaded by the Environmental Working Group (EWG) and targeted the chemical bisphenol A (BPA), a plastic and resin ingredient used to line metal food and drink cans. There are no government safety standards limiting the amount of BPA in canned food.
EWG's tests found:
- Of all foods tested, chicken soup, infant formula, and ravioli had BPA levels of highest concern. Just one to three servings of foods with these concentrations could expose a woman or child to BPA at levels that caused serious adverse effects in animal tests.
- For 1 in 10 cans of all food tested, and 1 in 3 cans of infant formula, a single serving contained enough BPA to expose a woman or infant to BPA levels more than 200 times the government's traditional safe level of exposure for industrial chemicals. The government typically mandates a 1,000- to 3,000-fold margin of safety between human exposures and levels found to harm lab animals, but these servings contained levels of BPA less than 5 times lower than doses that harmed lab animals.
|
BPA is a heavily produced industrial compound that has been detected in more than 2,000 people worldwide, including more than 95 percent of 400 people in the United States. More than 100 peer-reviewed studies have found BPA to be toxic at low doses, some similar to those found in people, yet not a single regulatory agency has updated safety standards to reflect this low-dose toxicity. FDA estimates that 17% of the U.S. diet comprises canned food; they last examined BPA exposures from food in 1996 but failed to set a safety standard.
Recommendations
BPA is associated with a number of health problems and diseases that are on the rise in the U.S. population, including breast and prostate cancer and infertility. Given widespread human exposure to BPA and hundreds of studies showing its adverse effects, the FDA and EPA must act quickly to set safe levels for BPA exposure based on the latest science on the low-dose toxicity of the chemical.
BPA is at unsafe levels in one of every 10 servings of canned foods (11%) and one of every 3 cans of infant formula (33%)
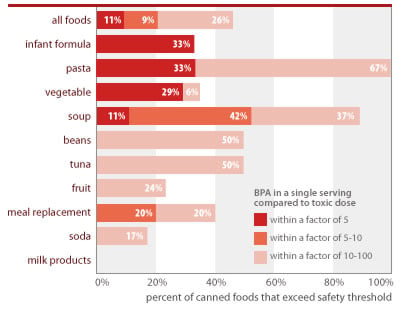
Source: Chemical analyses of 97 canned foods by Southern Testing and Research Division of Microbac Laboratories, Inc., North Carolina.
EWG calculated people's BPA exposures from canned food using the following assumptions: Calculations reflect a single adult serving, using label serving size and body weight of 60 kg (132 lbs); exposures for concentrated infant formula is calculated for exclusively formula-fed infant using average 3-month-old body weight (6 kg/13 lbs) and average daily formula ingestion (840 g/30 oz); formula is assumed diluted with water free of BPA. Estimated single-serving exposures are compared against BPA dose of 2 ug/kg/d linked in lab studies to permanent damage of reproductive system from in utero exposures and referenced as "toxic dose" in figure above (see Section 3 of this report).
Summary of findings
Widespread exposures, no safety standards. In studies conducted over the past 20 years, scientists have detected BPA in breast milk, serum, saliva, urine, amniotic fluid, and cord blood from at least 2,200 people in Europe, North America, and Asia (CERHR 2006). Researchers at the Centers for Disease Control and Prevention recently detected BPA in 95% of nearly 400 U.S. adults (Calafat et al. 2005). EWG-led biomonitoring studies have detected BPA in people from four states and the District of Columbia (EWG 2007). BPA ranks in the top two percent of high production volume chemicals in the U.S., with annual production exceeding a billion pounds (TSCA 2006), and is so common in products and industrial waste that it pollutes not only people but also rivers, estuaries, sediment, house dust, and even air nearly everywhere it is tested.
Yet despite its ubiquity and toxicity, BPA remains entirely without safety standards. It is allowed in unlimited amounts in consumer products, drinking water, and food, the top exposure source for most people. The lack of enforceable limits has resulted in widespread contamination of canned foods at levels that pose potential risks. For instance, analysis of our tests reveals that for one of every five cans tested, and for one-third of all vegetables and pastas (ravioli and noodles with tomato sauce), a single serving would expose a pregnant woman to BPA at levels that fall within a factor of 5 of doses linked to birth defects — permanent damage of developing male reproductive organs (Figure 1).
Many studies confirm BPA's low-dose toxicity across a diverse range of toxic effects
| Daily BPA exposure (ug/kg body weight-day) | CERHR conclusion* | Toxic effect | Study details | Reference | % cans tested by EWG with single-serving BPA levels within a margin of 10 from harmful dose |
|---|---|---|---|---|---|
| 0.0001 | not included | alterations in cell signalling pathways on the cell surface that control calcium eflux in cells | in-vitro study which compared activity of BPA and other hormone disruptors | Wozniak 2005 | 56.7 (all cans with detected BPA) |
| 0.025 | "very useful" | persistent changes to breast tissue, predisposes cells to hormones and carcinogens | fetal exposure, osmotic pumps, changes noted a 6 months of age | Muñoz-de-Toro 2005 | 55.7 |
| 0.025 | "useful and shows tissue effects at extremely low dose levels" | permanent changes to genital tract | fetal exposure, osmotic pumps | Markey 2005 | 55.7 |
| 0.2 | utility "limited" | decrease antioxidant enzymes | adult exposure, oral | Chitra 2003 | 47.4 |
| 0.25 | utility "to be added" | altered growth, cell size and lumen formation in mammary epithelium of mouse fetuses. | exposure during pregnancy w/osmotic pumps | Vandenberg 2007 | 45.4 |
| 2 | "useful" | increased prostate weight 30% | fetal exposure, oral route | Nagel 1997 | 20.6 |
| 2 | "moderately useful" | increased aggression at 8 weeks of life | fetal exposure, oral route | Kawai 2003 | 20.6 |
| 2.4 | "useful", but non-traditional endpoint | Decreased time from vaginal opening to first estrus, possibly earlier puberty | fetal exposure, oral route | Howdeshell 1999 | 17.5 |
| 2.4 | "useful" | lower bodyweight, increase of anogenital distance in both genders, signs of early puberty and longer estrus. | fetal exposure, oral route | Honma 2002 | 17.5 |
| 2.4 | "adequate" | decline in testicular testosterone | fetal and neonatal exposure, gavage | Akingbemi 2004 | 17.5 |
| 2.5 | utility "to be added" | breast cells predisposed to cancer | fetal exposure, osmotic pumps | Murray 2006 | 16.5 |
| 2.5 | not included | immune system impacts | oral exposure | Sawai 2003 | 16.5 |
| 10 | utility "very useful" | prostate cells more sensitive to hormones and cancer | infant oral exposure, 3 day duration | Ho 2006 | 2.1 |
| 10 | utility "very useful" | prostate cells more sensitive to hormones and cancer | fetal exposure, oral route, short duration | Timms 2005 | 2.1 |
| 10 | not included | insulin resistance develops in 2 days, chronic hyperinsulinemia at day 4 | subcutaneous injection, short duration exposure | Alonso-Magdalena 2006 | 2.1 |
| 10 | "very useful" | decreased maternal behaviors | fetal and neonatal exposure, oral route | Palanza 2002 | 2.1 |
| 20 | not included | damage to eggs and chromosomes | fetal exposure, osmotic pumps | Hunt 2003 | 0 |
| 20 | not included | damage to eggs | fetal exposure, osmotic pumps | Susiarjo 2007 | 0 |
| 20 | not included | brain effects - disrupted neocortical development by accelerating neuronal differentiation and migration | single injection | Nakamura 2006 | 0 |
| 30 | "...adequate for the evaluation process and gives cause for concern" | reversed the normal sex differences in brain structure and behavior | oral during gestation and lactation | Kubo 2003 | 0 |
| 30 | "suitable" | hyperactivity | oral | Ishido 2004 | 0 |
| 50 | EPA RfD | EPA's 'safe exposure level, based on outdated, high dose studies and a 1000-fold margin of safety | EPA 1998 | 0 |
*CERHR conclusion refers to the Center for Evaluation of Risks to Human Reproduction expert panel assessment of the utility of the study in the panel's review of BPA risks to human reproduction (CERHR 2006).
Statistics on percent cans with single servings that would yield human dose within a margin of 10 of the toxic dose are generated with the following assumptions: BPA calculations reflect a single adult serving, using label serving size and body weight of 60 kg (132 lbs); exposures for concentrated infant formula is calculated for exclusively formula-fed infant using average 3-month-old body weight (6 kg/13 lbs) and average daily formula ingestion (840 g/30 oz); formula is assumed diluted with water free of BPA.
BPA concentrations are expressed in parts per billion (ppb) by weight (micrograms of BPA per kilogram of food).
* Average is the geometric mean. Non-detects considered to be 1/2 the detection limit (1 ppb) for purposes of this calculation.
Government assessments fail to consider BPA low-dose toxicity. As of December 2004, 94 of 115 peer-reviewed studies had confirmed BPA's toxicity at low levels of exposure. At some of the very lowest doses the chemical causes permanent alterations of breast and prostate cells that precede cancer, insulin resistance (a hallmark trait of Type II diabetes), and chromosomal damage linked to recurrent miscarriage and a wide range of birth defects including Down's syndrome (vom Saal and Hughes 2005). Few chemicals have been found to consistently display such a diverse range of harm at such low doses.
Yet all of the most recent government reviews of bisphenol A have failed to set safety standards consistent with the chemical's low-dose toxicity. Each one either preceded the development of the low-dose literature, or heavily weighted industry-sponsored studies that are now known to have fundamental design flaws rendering them incapable of detecting BPA toxicity. U.S. safety reviews are described below:
- The U.S. EPA established its generic safety standard for BPA (the reference dose, or RfD) in 1987, a decade before the BPA low-dose literature was established (EPA 1987). The vast majority of studies finding BPA toxic at low doses have been published since 1997, the year that a pivotal study showed BPA's ability to harm the prostate at levels far below what was thought safe (vom Saal et al. 1997). EPA's safety standard is 25 times the dose now known to cause birth defects in lab studies (50 ug/kg/d vs. 2 ug/kg/d), and has not been updated for 20 years.
- The U.S. National Toxicology Program's 2001 assessment, which found BPA safe at low doses, relied heavily on industry-sponsored studies showing no low-dose BPA effects (NTP 2001). These studies are now known to have used animals resistant to the effects of estrogen-like chemicals such as BPA (vom Saal and Hughes 2005). The NTP assessment considered studies published in 2000 or earlier. The six years following this review have seen the publication of dozens of low-dose BPA studies that substantially bolster the now near irrefutable evidence for low-dose effects.
- FDA published estimates of infant and adult BPA exposures 10 years ago. Even though the Agency did not then and has not since assessed the low-dose toxicity of BPA, in 2005 an FDA official asserted, in response to questions from a California legislator considering a state BPA phase-out bill, that "...FDA sees no reason to change [its] long-held position that current [BPA] uses with food are safe" (FDA 2005). FDA makes this assertion even though the Agency has not yet established an Acceptable Daily Intake (ADI) for BPA, and has not even conducted the Agency's standard, basic toxicology study to determine a safe dose for humans (FDA 2007).
BPA's low dose toxicity. Companies began using BPA in metal can linings in the 1950s and 1960s (Schaefer and Simat 2004), fully twenty years after the chemical was first understood to be toxic (Dodds and Lawson, 1936 and 1938). These early warnings of toxicity were ignored or forgotten while companies steadily increased their reliance on BPA until it reached an annual U.S. production exceeding one billion pounds around 1990. In 1993 the chemical's signature toxic property, its ability to mimic estrogen, was accidentally discovered in a failed lab experiment (Krishnan et al. 1993), and the intervening years have witnessed the development of a body of low-dose science that has transformed our understanding of chemical toxicity.
Bisphenol A demonstrates the fallacy of nearly every long-standing tenet of government-style safety standards and traditional high-dose toxicology:
- Low doses and toxicity. Where traditional toxicology asserts that higher doses confer greater harm, bisphenol A tests show that low doses can be the most toxic of all, below the radar screen of the body's compensatory detoxifying mechanisms, or below overtly toxic doses that destroy the tissues under study. In one investigation a low dose of BPA produced a 70% higher growth rate of prostate cancer cells in lab animals than did higher doses (Wetherill et al. 2002). In another study lower doses of BPA resulted in higher rates of breast cell growth that can precede cancer (Markey et al. 2001). ("Low doses" are typically defined as those that produce tissue concentrations at or below those in the typical range of human exposures.)
- Timing of the dose. While traditional methods set safety standards to control risks defined in adulthood, bisphenol A studies reveal that exposures at other times can confer far higher risks, especially in the womb and during early childhood. For example, recent studies show that prenatal exposure to BPA causes breast cancer in adult rats (Murray et al. 2006), and causes genetic changes resulting in greater risk of prostate cancer in later life. (Ho et al. 2006). In another study adult rats which had been dosed in the womb developed breast cancer in adulthood (Munoz-de-Toro 2005); these exposure levels during adulthood would not have caused cancer.
- Genetic susceptibility. Traditional toxicology holds that a chemical's potency and risks are constant, regardless of who is exposed. Bisphenol A suggests a different truth: A person's genetics plays an important role in defining risks and health outcomes from exposures to toxic chemicals. For instance, studies suggest that for some but not all babies, BPA accumulates in amniotic fluid, suggesting differing innate capacities for excretion that would be defined by genetics (Yamada et al. 2002). A recent study of mammary gland development showed that animals exposed to BPA in utero are more likely to develop mammary tumors when they are exposed to carcinogenic chemicals later in life, compared to animals not previously exposed to BPA (Durando et al. 2007). This study is one of many suggesting that early-life exposures to BPA may alter the expression or strength of genes to dramatically alter disease risk later in life.
Over the past year an average of four new BPA toxicity studies have been published in the peer-reviewed literature every month. New discoveries on BPA surface so routinely that the CERHR review document (CERHR 2006) describes fully 465 studies conducted primarily over the past 14 years. Among recent works:
- A study showing that BPA exposures lead to an error in cell division called aneuploidy that causes spontaneous miscarriages, cancer, and birth defects in people, including Down Syndrome (Hunt et al. 2003).
- An investigation demonstrating that low doses of BPA spur both the formation and growth of fat cells, the two factors that drive obesity in humans (Masumo et al. 2002).
- A study linking low doses of BPA to insulin resistance, a risk factor for Type II diabetes (Alonso-Magdalena et al. 2006).
- A preliminary investigation linking BPA exposures to recurrent miscarriage in a small group of Japanese women, made potentially pivotal by its concordance with lab studies of BPA-induced chromosome damage that could well cause miscarriage (Sugiura-Ogasawara 2005).
The unusually broad toxicity of BPA is explained by a prominent scientist as stemming from the fact that BPA can alter the behavior of over 200 genes — more than one percent of all human genes (Myers 2006). These genes control the growth and repair of nearly every organ and tissue in the body. Taken in its totality, the range of toxic effects linked to BPA is startlingly similar to the litany of human health problems on the rise or common across the population, including breast and prostate cancer, diabetes, obesity, infertility, and polycystic ovarian syndrome (Myers 2007).
Studies show that BPA is toxic to lab animals at doses overlapping with or very near to human exposures, and that the chemical causes toxic effects that are on the rise or very common in people. These disturbing facts raise questions about the extent to which current, widespread exposures to BPA are contributing to the burden of human disease.
Were the federal government to develop safety standards reflecting any of the more than 200 low-dose studies of BPA toxicity, the chemical would become the first widespread industrial compound with a government-recognized, harmful dose at such remarkably low levels that in some cases appear to overlap with human exposures. The science would fully justify a strict safety standard and would force industry to change food packaging to dramatically decrease the widespread BPA exposures to which they are currently subjecting the public.
FDA fails to protect the public. FDA is responsible for ensuring that food packaging chemicals like BPA are safe. In the case of BPA, the Agency has deemed the chemical safe even though its own exposure estimates for infants exceed doses shown to permanently harm the developing male reproductive system.
FDA does not restrict BPA levels in food. In the wake of a 1993 experiment proving that BPA disrupts estrogen levels, FDA tested 14 cans of infant formula and a few foods that adults eat, calculated exposures from these tests, and found them to be within safe levels (CERHR 2006). To make this determination the Agency compared the estimated exposures to "safe" doses far higher than those now known to cause permanent harm to lab animals.
Dr. George Pauli, at the time FDA's associate director for science and policy, offered this rationale: "FDA sees no reason at this time to ban or otherwise restrict the uses now in practice" (Pauli 2005). Never mind that the Agency's estimated exposures for infants, at 15-24 ug/kg/d, exceed by a factor of up to 10 the dose shown to permanently alter prostate gland growth.
Bisphenol A is just one of hundreds of chemicals that pollute people - proof of critical need to reform our system of public health protections. Studies by European scientists show that BPA is just one of many chemicals that leach out of food can linings. Tests of just three can coatings found at least 23 different BPA-related chemicals leaching into food, all without legal limits (Schaefer and Simat 2004). Research shows these contaminants occur at levels that can dwarf better-known environmental pollutants that accumulate in food, like PCBs and DDT. One scientist writes that "Concentrations of [migrant chemicals like BPA] commonly exceed...pesticides by orders of magnitude; most of the migrating compounds are not even identified; and only a few have been tested for toxicity..." (Grob et al. 1999).
FDA has tallied more than 1,000 indirect food additive chemicals in packaging and food processing, but food is just one of the many ways humans are exposed to industrial chemicals. EWG research reveals more than 200 pollutants in tap water supplies across the country; thousands of chemicals in cosmetics and personal care products; 470 industrial chemicals and pesticides in human tissues; and an average of 200 pollutants in each of 10 babies tested at the moment of birth. Nothing is known about the safety of the complex mixtures of low doses of a myriad of industrial chemicals in the human body.
The nation's system of public health protections from industrial chemicals like BPA are embodied in the Toxic Substances Control Act, a law passed in 1976 that is the only major environmental or public health statute that has never been updated. Under this law companies are not required to test chemicals for safety before they are sold and are not required to track whether their products end up in people at unsafe levels. As a result of this broken system, BPA is now one of the most widely used industrial chemicals, is found at unsafe levels in people, is allowed in unlimited quantities in a broad range of consumer products, and is entirely without safety standards. BPA gives irrefutable proof that our system of public health protections must be strengthened to protect children and others most vulnerable to chemical harm.
Canned food test results
Canned foods are thought to be the predominate route of BPA exposure (CERHR 2006). Numerous studies support this fact, including an investigation of BPA exposures for 257 young children in North Carolina and Ohio day care centers. Researchers collected samples of the air, water, dust, hand wipes and the daily diet and attributed 99 percent of children's daily BPA exposures to food (Wilson, Chuang et al. 2003; Wilson, Chuang et al. 2007). Despite this fact, very little canned food testing has been performed. Both the Plastics Industry and FDA have based their safety or exposure assessments for BPA on incredibly few canned food tests, fewer than 20 in both cases (Allan B. Bailey 1996; SPI 2007).
EWG tested foods and beverages from nearly 100 cans purchased in grocery stores in 3 states. EWG tested 28 different types of foods including canned fruits, vegetables, pasta, beans, infant formula, meal replacements and canned milk. We tested 1 to 6 samples of each type food. BPA levels varied from less the detection limit to a maximum level of 385 micrograms BPA per kilogram food (a part per billion). BPA test results for individual cans are shown at the end of this section.
Many studies confirm BPA's low-dose toxicity across a diverse range of toxic effects
| Daily BPA exposure (ug/kg body weight-day) | CERHR conclusion* | Toxic effect | Study details | Reference | % cans tested by EWG with single-serving BPA levels within a margin of 10 from harmful dose |
|---|---|---|---|---|---|
| 0.0001 | not included | alterations in cell signalling pathways on the cell surface that control calcium eflux in cells | in-vitro study which compared activity of BPA and other hormone disruptors | Wozniak 2005 | 56.7 (all cans with detected BPA) |
| 0.025 | "very useful" | persistent changes to breast tissue, predisposes cells to hormones and carcinogens | fetal exposure, osmotic pumps, changes noted a 6 months of age | Munoz-de-Toro 2005 | 55.7 |
| 0.025 | "useful and shows tissue effects at extremely low dose levels" | permanent changes to genital tract | fetal exposure, osmotic pumps | Markey 2005 | 55.7 |
| 0.2 | utility "limited" | decrease antioxidant enzymes | adult exposure, oral | Chitra 2003 | 47.4 |
| 0.25 | utility "to be added" | altered growth, cell size and lumen formation in mammary epithelium of mouse fetuses. | exposure during pregnancy w/osmotic pumps | Vandenberg 2007 | 45.4 |
| 2 | "useful" | increased prostate weight 30% | fetal exposure, oral route | Nagel 1997 | 20.6 |
| 2 | "moderately useful" | increased aggression at 8 weeks of life | fetal exposure, oral route | Kawai 2003 | 20.6 |
| 2.4 | "useful", but non-traditional endpoint | Decreased time from vaginal opening to first estrus, possibly earlier puberty | fetal exposure, oral route | Howdeshell 1999 | 17.5 |
| 2.4 | "useful" | lower bodyweight, increase of anogenital distance in both genders, signs of early puberty and longer estrus. | fetal exposure, oral route | Honma 2002 | 17.5 |
| 2.4 | "adequate" | decline in testicular testosterone | fetal and neonatal exposure, gavage | Akingbemi 2004 | 17.5 |
| 2.5 | utility "to be added" | breast cells predisposed to cancer | fetal exposure, osmotic pumps | Murray 2006 | 16.5 |
| 2.5 | not included | immune system impacts | oral exposure | Sawai 2003 | 16.5 |
| 10 | utility "very useful" | prostate cells more sensitive to hormones and cancer | infant oral exposure, 3 day duration | Ho 2006 | 2.1 |
| 10 | utility "very useful" | prostate cells more sensitive to hormones and cancer | fetal exposure, oral route, short duration | Timms 2005 | 2.1 |
| 10 | not included | insulin resistance develops in 2 days, chronic hyperinsulinemia at day 4 | subcutaneous injection, short duration exposure | Alonso-Magdalena 2006 | 2.1 |
| 10 | "very useful" | decreased maternal behaviors | fetal and neonatal exposure, oral route | Palanza 2002 | 2.1 |
| 20 | not included | damage to eggs and chromosomes | fetal exposure, osmotic pumps | Hunt 2003 | 0 |
| 20 | not included | damage to eggs | fetal exposure, osmotic pumps | Susiajro 2007 | 0 |
| 20 | not included | brain effects - disrupted neocortical development by accelerating neuronal differentiation and migration | single injection | Nakamura 2006 | 0 |
| 30 | "...adequate for the evaluation process and gives cause for concern" | reversed the normal sex differences in brain structure and behavior | oral during gestation and lactation | Kubo 2001 | 0 |
| 30 | "suitable" | hyperactivity | oral | Ishido 2004 | 0 |
| 50 | EPA RfD | EPA's 'safe exposure level, based on outdated, high dose studies and a 1000-fold margin of safety | EPA 1998 | 0 |
*CERHR conclusion refers to the Center for Evaluation of Risks to Human Reproduction expert panel assessment of the utility of the study in the panel's review of BPA risks to human reproduction (CERHR 2006).
Statistics on percent cans with single servings that would yield human dose within a margin of 10 of the toxic dose are generated with the following assumptions: BPA calculations reflect a single adult serving, using label serving size and body weight of 60 kg (132 lbs); exposures for concentrated infant formula is calculated for exclusively formula-fed infant using average 3-month-old body weight (6 kg/13 lbs) and average daily formula ingestion (840 g/30 oz); formula is assumed diluted with water free of BPA.
We found widespread contamination of BPA in canned foods. All six cans of spaghetti and ravioli tested contained measurable levels of BPA, averaging 63.5 parts per billion. Five of the six cans of baked beans examined had measurable levels of BPA, averaging 9.7 parts per billion. Two of six cans of infant formula tested contained BPA. The exposure that an infant might receive from canned formula, given his or her small size and limited food sources, makes the level of contamination in these cans particularly disturbing.
BPA is found in canned food around the world
Our study provides the most comprehensive U.S.-based examination of BPA in canned food available, but BPA contamination in food is a global concern. Below we show findings of other studies from around the world, as described in CERHR (2006).
Summary of BPA measurements in canned food from 9 previous studies
| Food type | Number of studies | Location | Total number of cans tested | Percent of cans with BPA detected | BPA range, ppb (ug/kg) | EWG study: BPA range, ppb (ug/kg) | Ref- erences |
|---|---|---|---|---|---|---|---|
| Beverages | 1 | Austria | 7 | 0% | <0.9 - 3.4 | 2.4 - 8.2 | [2] |
| Canned meat+ | 3 | New Zealand, UK | 10 | ~75% | 8.6 - 89 | NA | [5, 6, 9] |
| Fruit | 2 | Austria, UK | 6 | >80% | 5 - 38 | 2.2 - 27 | [2, 5] |
| Fruit & vegetables | 1 | New Zealand | 38 | unavailable | <20 - 24 | NA | [9] |
| Infant food | 2 | New Zealand, UK | 10 | 30% | <10 - 77 | NA | [5, 9] |
| Infant formula | 3 | US, UK, Taiwan | 24 | 80% | <0.002 - 113 | 10.9 - 17.1 | [1, 5, 7] |
| Pasta | 3 | New Zealand, UK | 10 | >50% | <7 - 130 | 16.2 - 247 | [5, 6, 9] |
| Soup | 3 | New Zealand, UK | 15 | unavailable | <2 - 39 | 8.6 - 385 | [5, 6, 9] |
| Tuna | 4 | New Zealand, UK, Mexico, Austria | 16 | 75% | <7 - 109 | 80 - 108 | [5, 8, 9, 10] |
| Vegetables | 5 | Austria, UK, Spain, US | 34 | >80% | 4 - 76 | 8.9 - 330 | [2, 3, 4, 5, 6] |
+ Does not include tuna
References
U.S.: [1] Biles, J. E., McNeal, T. P. and Begley, T. H. Determination of bisphenol A migrating from epoxy can coatings to infant formula liquid concentrates. J Agric Food Chem 1997; 45: 4697-4700.
Austria: [2] Braunrath, R., Podlipna, D., Padlesak, S. and Cichna-Markl, M. Determination of bisphenol A in canned foods by immunoaffinity chromatography, HPLC, and fluorescence detection. J Agric Food Chem 2005; 53: 8911-7.
Spain: [3] Brotons, J. A., Olea-Serrano, M. F., Villalobos, M., Pedraza, V. and Olea, N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect 1995; 103: 608-12.
U.S.: [4] FDA. Cumulative Exposure Estimated for Bisphenol A (BPA), Individually for Adults and Infants from Its Use in Epoxy-Based Can Coatings and Polycarbonate (PC) Articles, verbal request of 10-23-95, memorandum to G. Diachenki, Ph.D, Division of Product Manufacture and Use, HGS-245, from Allan B. Bailey, Ph.D., Chemistry Review Branch, HFS-245. Department of Health and Human Services, Food and Drug Administration. Food and Drug Administration; 1996.
U.K.: [5] Goodson, A., Robin, H., Summerfield, W. and Cooper, I. Migration of bisphenol A from can coatings--effects of damage, storage conditions and heating. Food Addit Contam 2004; 21: 1015-26.
U.K.: [6] Goodson, A., Summerfield, W. and Cooper, I. Survey of bisphenol A and bisphenol F in canned foods. Food Addit Contam 2002; 19: 796-802.
Taiwan: [7] Kuo, H.-W. and Ding, W.-H. Trace determination of bisphenol A and phytoestrogens in infant formula powders by gas chromatography-mass spectometry. J Chromatogr A 2004; 1027: 67-74.
Mexico: [8] Munguía-López , E. M., Gerardo-Lugo, S., Peralta, E., Bolumen, S. and Soto-Valdez, H. Migration of bisphenol A (BPA) from can coatings into a fatty-food simulant and tuna fish. Food Addit Contam 2005; 22: 892-8
New Zealand: [9] Thomson, B. M. and Grounds, P. R. Bisphenol A in canned foods in New Zealand: an exposure assessment. Food Addit Contam 2005; 22: 65-72.
Summary of BPA measurements in canned food from 9 previous studies
| Food type | Number of studies | Location | Total number of cans tested | Percent of cans with BPA detected | BPA range, ppb (ug/kg) | EWG study: BPA range, ppb (ug/kg) | Ref- erences |
|---|---|---|---|---|---|---|---|
| Beverages | 1 | Austria | 7 | 0% | <0.9 - 3.4 | 2.4 - 8.2 | [2] |
| Canned meat+ | 3 | New Zealand, UK | 10 | ~75% | 8.6 - 89 | NA | [5, 6, 9] |
| Fruit | 2 | Austria, UK | 6 | >80% | 5 - 38 | 2.2 - 27 | [2, 5] |
| Fruit & vegetables | 1 | New Zealand | 38 | unavailable | <20 - 24 | NA | [9] |
| Infant food | 2 | New Zealand, UK | 10 | 30% | <10 - 77 | NA | [5, 9] |
| Infant formula | 3 | US, UK, Taiwan | 24 | 80% | <0.002 - 113 | 10.9 - 17.1 | [1, 5, 7] |
| Pasta | 3 | New Zealand, UK | 10 | >50% | <7 - 130 | 16.2 - 247 | [5, 6, 9] |
| Soup | 3 | New Zealand, UK | 15 | unavailable | <2 - 39 | 8.6 - 385 | [5, 6, 9] |
| Tuna | 4 | New Zealand, UK, Mexico, Austria | 16 | 75% | <7 - 109 | 80 - 108 | [5, 8, 9, 10] |
| Vegetables | 5 | Austria, UK, Spain, US | 34 | >80% | 4 - 76 | 8.9 - 330 | [2, 3, 4, 5, 6] |
+ Does not include tuna
References
U.S.: [1] Biles, J. E., McNeal, T. P. and Begley, T. H. Determination of bisphenol A migrating from epoxy can coatings to infant formula liquid concentrates. J Agric Food Chem 1997; 45: 4697-4700.
Austria: [2] Braunrath, R., Podlipna, D., Padlesak, S. and Cichna-Markl, M. Determination of bisphenol A in canned foods by immunoaffinity chromatography, HPLC, and fluorescence detection. J Agric Food Chem 2005; 53: 8911-7.
Spain: [3] Brotons, J. A., Olea-Serrano, M. F., Villalobos, M., Pedraza, V. and Olea, N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect 1995; 103: 608-12.
U.S.: [4] FDA. Cumulative Exposure Estimated for Bisphenol A (BPA), Individually for Adults and Infants from Its Use in Epoxy-Based Can Coatings and Polycarbonate (PC) Articles, verbal request of 10-23-95, memorandum to G. Diachenki, Ph.D, Division of Product Manufacture and Use, HGS-245, from Allan B. Bailey, Ph.D., Chemistry Review Branch, HFS-245. Department of Health and Human Services, Food and Drug Administration. Food and Drug Administration; 1996.
U.K.: [5] Goodson, A., Robin, H., Summerfield, W. and Cooper, I. Migration of bisphenol A from can coatings--effects of damage, storage conditions and heating. Food Addit Contam 2004; 21: 1015-26.
U.K.: [6] Goodson, A., Summerfield, W. and Cooper, I. Survey of bisphenol A and bisphenol F in canned foods. Food Addit Contam 2002; 19: 796-802.
Taiwan: [7] Kuo, H.-W. and Ding, W.-H. Trace determination of bisphenol A and phytoestrogens in infant formula powders by gas chromatography-mass spectometry. J Chromatogr A 2004; 1027: 67-74.
Mexico: [8] Munguía-López , E. M., Gerardo-Lugo, S., Peralta, E., Bolumen, S. and Soto-Valdez, H. Migration of bisphenol A (BPA) from can coatings into a fatty-food simulant and tuna fish. Food Addit Contam 2005; 22: 892-8
New Zealand: [9] Thomson, B. M. and Grounds, P. R. Bisphenol A in canned foods in New Zealand: an exposure assessment. Food Addit Contam 2005; 22: 65-72.
BPA is toxic at low doses
Numerous studies indicate exposure to low levels of BPA causes a range of serious health effects in laboratory animals, particularly when exposures occur in utero (Maffini 2006). Below we list 21 key studies that indicate low-dose effects. Many of these were deemed by CERHR to be 'useful' for the purposes of evaluating BPA's low-dose effects on human health (CERHR 2006). The harmful doses defined by these studies are well below EPA's current safe dose for BPA of 50 ug/kg-day. And as shown on the table below, a pregnant woman's or infant's BPA dose from a single serving of food from many of the cans tested in our study would fall within a margin of 10 from the harmful effects shown in these studies.
Many studies confirm BPA's low-dose toxicity across a diverse range of toxic effects
| Daily BPA exposure (ug/kg body weight-day) | CERHR conclusion* | Toxic effect | Study details | Reference | % cans tested by EWG with single-serving BPA levels within a margin of 10 from harmful dose |
|---|---|---|---|---|---|
| 0.0001 | not included | alterations in cell signalling pathways on the cell surface that control calcium eflux in cells | in-vitro study which compared activity of BPA and other hormone disruptors | Wozniak 2005 | 56.7 (all cans with detected BPA) |
| 0.025 | "very useful" | persistent changes to breast tissue, predisposes cells to hormones and carcinogens | fetal exposure, osmotic pumps, changes noted a 6 months of age | Muñoz-de-Toro 2005 | 55.7 |
| 0.025 | "useful and shows tissue effects at extremely low dose levels" | permanent changes to genital tract | fetal exposure, osmotic pumps | Markey 2005 | 55.7 |
| 0.2 | utility "limited" | decrease antioxidant enzymes | adult exposure, oral | Chitra 2003 | 47.4 |
| 0.25 | utility "to be added" | altered growth, cell size and lumen formation in mammary epithelium of mouse fetuses. | exposure during pregnancy w/osmotic pumps | Vandenberg 2007 | 45.4 |
| 2 | "useful" | increased prostate weight 30% | fetal exposure, oral route | Nagel 1997 | 20.6 |
| 2 | "moderately useful" | increased aggression at 8 weeks of life | fetal exposure, oral route | Kawai 2003 | 20.6 |
| 2.4 | "useful", but non-traditional endpoint | Decreased time from vaginal opening to first estrus, possibly earlier puberty | fetal exposure, oral route | Howdeshell 1999 | 17.5 |
| 2.4 | "useful" | lower bodyweight, increase of anogenital distance in both genders, signs of early puberty and longer estrus. | fetal exposure, oral route | Honma 2002 | 17.5 |
| 2.4 | "adequate" | decline in testicular testosterone | fetal and neonatal exposure, gavage | Akingbemi 2004 | 17.5 |
| 2.5 | utility "to be added" | breast cells predisposed to cancer | fetal exposure, osmotic pumps | Murray 2007 | 16.5 |
| 2.5 | not included | immune system impacts | oral exposure | Sawai 2003 | 16.5 |
| 10 | utility "very useful" | prostate cells more sensitive to hormones and cancer | infant oral exposure, 3 day duration | Ho 2006 | 2.1 |
| 10 | utility "very useful" | prostate cells more sensitive to hormones and cancer | fetal exposure, oral route, short duration | Timms 2005 | 2.1 |
| 10 | not included | insulin resistance develops in 2 days, chronic hyperinsulinemia at day 4 | subcutaneous injection, short duration exposure | Alonso-Magdalena 2006 | 2.1 |
| 10 | "very useful" | decreased maternal behaviors | fetal and neonatal exposure, oral route | Palanza 2002 | 2.1 |
| 20 | not included | damage to eggs and chromosomes | fetal exposure, osmotic pumps | Hunt 2003 | 0 |
| 20 | not included | damage to eggs | fetal exposure, osmotic pumps | Susiarjo 2007 | 0 |
| 20 | not included | brain effects - disrupted neocortical development by accelerating neuronal differentiation and migration | single injection | Nakamura 2006 | 0 |
| 30 | "...adequate for the evaluation process and gives cause for concern" | reversed the normal sex differences in brain structure and behavior | oral during gestation and lactation | Kubo 2003 | 0 |
| 30 | "suitable" | hyperactivity | oral | Ishido 2004 | 0 |
| 50 | EPA RfD | EPA's 'safe exposure level, based on outdated, high dose studies and a 1000-fold margin of safety | EPA 1998 | 0 |
*CERHR conclusion refers to the Center for Evaluation of Risks to Human Reproduction expert panel assessment of the utility of the study in the panel's review of BPA risks to human reproduction (CERHR 2006).
Statistics on percent cans with single servings that would yield human dose within a margin of 10 of the toxic dose are generated with the following assumptions: BPA calculations reflect a single adult serving, using label serving size and body weight of 60 kg (132 lbs); exposures for concentrated infant formula is calculated for exclusively formula-fed infant using average 3-month-old body weight (6 kg/13 lbs) and average daily formula ingestion (840 g/30 oz); formula is assumed diluted with water free of BPA.
People are exposed at harmful levels
A recent study from the Centers for Disease Control tested a demographically diverse group of almost 400 Americans for evidence of exposure to BPA and found that 95% of study participants had the chemical in their urine (Calafat et al. 2005). BPA has been linked to a variety of health outcomes which are increasing in the United States and responsible for a major toll on our collective health. These include breast and prostate cancer, and infertility (Maffini 2006).
An analysis of CDC's body burden measurements shows that women are routinely exposed within a margin of 10 to doses that caused toxic effects in laboratory studies. The government typically mandates a 1,000- to 3,000-fold margin of safety between human exposures and levels found to harm lab animals. In the case of BPA, however, women routinely exceed this safety margin for 7 of the toxic doses from studies the CERHR has classified as appropriate for assessing human risks (see CERHR 2006 and Section 3 of this report). An analysis of CDC's data on women's exposures to BPA shows:
- 90% of all women are exposed to BPA at levels within a factor of 10 or less from doses shown to increase breast cancer risk and cause permanent changes in genital tract formation (see Section 3 for details). Scientists are debating the appropriate "effective dose" of BPA from the particular studies that measured these toxic effects, since BPA was delivered directly to the animals' bloodstream instead of through ingestion.
- 1.1% of all women are exposed to BPA within a margin of 10 of doses linked to early puberty.
- 3.1% of all women are exposed to BPA within a margin of 10 of doses linked to damage to the developing male reproductive system.
CDC data show that people are routinely exposed to unsafe levels of BPA
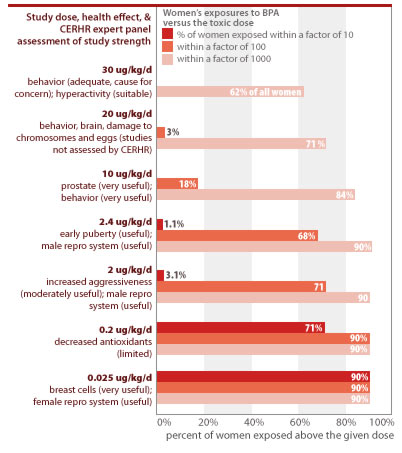
Source: EWG analysis of CDC measurements of BPA in urine from Calafat et al. (2005). Estimates assume 2 liters of urine excreted per day; and rely on linear interpolation between the percentiles of data provided in the study documentation, and linear extrapolation above the 95th percentile, using a best-fit estimation from the intercept at the 100th percentile of exposure.
Populations with unusual exposures are at special risk
Body burden studies indicate a fraction of the population is highly exposed to BPA. The most highly exposed people in the adult monitoring study excreted 6 times more BPA than the average participant (Calafat 2005). A study of 7-year-old girls from 5 US cities found similar, if not slightly higher, exposures for children compared to adults (Wolff et al. 2006). Only summary results are available from the Wolff study, but they indicate an average exposure of 0.06 ug/kg-day, (CERHR 2006) and a maximum of 54.3 ug/L, 27 times higher than the median concentration.
Since both studies collected samples at a single point in time it is difficult to know how much an individual's exposure varies from day to day. A few studies have collected 24-hour urine measurements over several days and found a high degree of variation in day-to-day exposure for individuals (Arakawa 2004).
Pre-natal and early life exposures. Studies have also documented BPA in fetal cord blood, amniotic fluid, and breast milk in women from industrialized countries, sometimes at higher concentrations than in maternal serum (Ikezuki et al. 2002; Schonfelder, Flick et al. 2002; Schonfelder, Wittfoht et al. 2002; Sun 2004; Irie et al. 2004; Kuruto-Niwa, Tateoka et al. 2007).
In laboratory animals BPA is rapidly passed to the developing fetus, and detected at higher concentrations in fetal than maternal blood (Schonfelder, Flick et al. 2002; Schonfelder, Wittfoht et al. 2002). Concern about daily pre- and post-natal exposures is heightened by the fact that detoxification mechanisms that rapidly deactivate and filter BPA from the body are not fully functional in the fetus and newborn. Most BPA is detoxified through a process known as glucuronidation, and the body's glucuronidation systems are not fully developed at birth (Schonfelder, Flick et al. 2002; Schonfelder, Wittfoht et al. 2002).
Takahashi exposed pregnant rats to BPA and found both the mean and maximum retention times of BPA in fetuses are longer than in maternal blood. (Takahashi and Oishi 2000) Hepatic glucuronidation activity in children aged 13–24 months was found to be 12- to 40-fold lower than in adults for five pharmaceutical drugs (Strassburg, Strassburg et al. 2002).
These findings provide evidence that exposure to BPA is pervasive and inclusive of the most vulnerable members of the population, namely the developing fetus, infant, and child. The body's immature detoxification systems result in greater exposure to the harmful form of BPA during infancy — a vulnerable period for brain and reproductive system development.
EWG's measurements of infant formula and various tests finding BPA leaching from polycarbonate baby bottles indicate that heightened pre-natal exposures might be followed by an intense period of dietary BPA exposure, resulting in much greater vulnerability for infants as opposed to children and adults.
Additional sources of BPA exposure in the human population
Canned food is a predominant, but not exclusive source of daily BPA exposure. BPA is found in many everyday products such as the hard clear plastic food containers — including baby bottles, baby toys, dental fillings and sealants, electronics, adhesives, paints and varnishes. BPA is found in a variety of other PVC plastics. Brominated BPA is a fire retardant with widespread use in the plastics used for electronics.
Polycarbonate plastics are rigid and clear or translucent, and often used for foods since they do not impart a plastic taste into food products. Polycarbonate plastics are often marked with the number 7. They are common in baby bottles, sippy toddler cups, Nalgene and other adult water bottles as well as plastics designed for longer-term food storage and microwave use. Water services using carboys also employ polycarbonate plastic. Polycarbonate is also made into disposable plastic tableware. Migration studies show a small degree of BPA leaching from plastics that are heated or abraded.
Another common source of BPA exposure is from tooth-colored dental fillings or sealants — which can contain up to 50 percent BPA (FDA 2004). Most exposure assessments consider these exposures to be intense but short lived since BPA is rapidly excreted from the body.
BPA is used in a variety of industrial products most of which result in little exposure for the general population. However worker exposures in these settings would be a particular concern for the smaller number of people with on-going, high level exposures. These might include plastics manufacturing for mobile phone housings, displays, computer parts, household electrical equipment, lamp fittings, automotive plastics, thermal paper and printing inks (CERHR 2006).
BPA is a building block for polycarbonate plastic and epoxy resins. BPA and related compounds leach from plastic and metal can linings into food and drinks — particularly after heating or as plastic ages — and from dental sealants.
Canned food exposures are significant
An analysis of EWG's tests for BPA contamination in canned food reveals that people who eat canned foods are likely to ingest doses of BPA that are very close to levels now known to harm laboratory animals. EWG assessed human exposures in two ways - estimating single-day exposures using standard government assumptions for consumption and body weight; and estimating chronic exposures for women who routinely eat canned food, via Monte Carlo techniques and government data and assumptions on relative consumption rates for different types of canned foods. Our analyses show:
- Single serving exposures. For 1 in 10 cans of all food tested, and 1 in 3 cans of infant formula, a single serving contained enough BPA to expose a woman or infant to BPA levels more than 200 times the government's traditional safe level of exposure for industrial chemicals (Figure). The government typically mandates a 1,000- to 3,000-fold margin of safety between human exposures and levels found to harm lab animals, but these servings contained levels of BPA less than 5 times lower than doses that harmed lab animals.
- Chronic exposures. Our analyses show that for women who routinely eat canned food, chronic exposure levels throughout pregnancy can exceed safe doses. For example, the BPA dose for one-quarter of all women eating 2 servings of canned food daily would fall within a margin of 10 from levels linked to prostate damage and diabetes in studies of in utero exposures.
Methods and findings from our in-depth analysis of BPA exposures for women and infants are described in detail below.
Single serving exposures to canned foods contaminated with BPA
Our analysis shows that single servings from 20 of the 53 cans with detectable BPA put consumers within an uncomfortable range of the levels that directly harm lab animals. These tests found levels just 1.6 to 10 times lower than the doses that impacted the male reproductive system and caused increased aggressiveness in lab animals (2 ug/kg-day) (Nagel 1998; Kawai 2003). In comparison, regulatory agencies typically require a margin of safety of 1000 to 3000 between human exposures and the effects found in animal studies. Methods of analysis are described below the figure that displays exposure findings for each food type we tested.
While most of the available BPA toxicity studies dose lab animals over longer durations than a single day, short-term or every single day doses such as those estimated below can be significant when they occur in windows of vulnerability during development.
BPA is at unsafe levels in one of every 10 servings of canned foods (11%) and one of every 3 cans of infant formula (33%)

Source: Chemical analyses of 97 canned foods by Southern Testing and Research Division of Microbac Laboratories, Inc., North Carolina.
EWG calculated people's BPA exposures from canned food using the following assumptions: Calculations reflect a single adult serving, using label serving size and body weight of 60 kg (132 lbs); exposures for concentrated infant formula is calculated for exclusively formula-fed infant using average 3-month-old body weight (6 kg or 13 lbs) and average daily formula ingestion (840 g or 30 oz); formula is assumed diluted with water free of BPA. Estimated single-serving exposures are compared against BPA dose of 2 ug/kg/d linked in lab studies to permanent damage of reproductive system from in utero exposures and referenced as "toxic dose" in figure above (see Section 3 of this report).
Government safety standards are often set to control human exposures at least a factor of 1,000 below the harmful effects in animal studies. This margin of safety is incorporated because public health agencies are aiming to protect, in the case of a developmental toxicant like BPA, four million pregnancies each year based on data from which is typically a small number of laboratory animals. Agencies normally incorporate safety factors to account for intra- and interspecies differences (rat-to-rat differences in susceptibility, and rat-to-human differences, for instance) as well as factors to account for data gaps and other uncertainties.
In the case of BPA toxicity, the lowest exposure of 2 to 2.5 ug per kilogram of body weight per day via food or water shows permanent effects to reproductive systems, antioxidant hormones, behavior and hormone levels (Nagel 1997; Chitra 2003; Kawai 2003; Howdeshell 1999; Honma 2002). With these lowest-dose studies in mind, we tallied the percent of our canned food samples that would subject an average consumer to an unacceptable risk of harm. The exposures reflected below for infant formula would be even higher for children younger, lighter, or hungrier than the typical 3-month-old assumed for our assessment.
We tallied the percent of cans by food type that contain servings of BPA-contaminated food that would fall within a margin of 5 to 100 of the 2.0 ug/kg-d BPA dose linked to permanent effects in laboratory animals. All the canned pasta and half the canned tuna and beans tested contain levels of BPA in a single serving that would exceed a level of exposure calculated using a margin of safety of 100, or an exposure level 100 times less than that associated with harmful health effects in laboratory studies. One-third of the canned infant formula tested contained levels of BPA in a single serving that would exceed a level of exposure calculated using a margin of safety of just 5.
BPA in a single serving of many foods tested would exceed a minimal margin of safety from the low dose effects of oral exposure (2.0 ug/kg-d)+
| Food Type | Number of cans tested | Percent of cans with single-serving dose within margin of 5 from harmful level | Percent of cans with single-serving dose within margin of 10 from harmful dose | Percent of cans with single-serving dose within margin of 100 from harmful dose | High-end daily intake for consumer* (ug/kg-d) |
|---|---|---|---|---|---|
| Pasta | 6 | 33% | 33% | 100% | 0.87 |
| Infant formula# | 6 | 33% | 33% | 33% | 1.20 |
| Vegetable | 17 | 29% | 29% | 35% | 0.65 |
| Soup | 19 | 11% | 53% | 89% | 1.32 |
| Meal replacement | 5 | 0% | 20% | 40% | 0.34 |
| Tuna | 6 | 0% | 0% | 50% | 0.12 |
| Beans | 6 | 0% | 0% | 50% | 0.08 |
| Fruit | 17 | 0% | 0% | 24% | 0.06 |
| Soda | 12 | 0% | 0% | 17% | 0.05 |
| Milk products | 3 | 0% | 0% | 0% | 0.004 |
| All foods | 97 | 11% | 21% | 46% | NA |
*Calculated for a single serving of the can with the maximum BPA detection for that food type
# Serving = average daily intake for 3 month-old infant exclusively formula fed
+ Nagel et al. 1997
The most comprehensive assessments of BPA exposure are body burden studies of adults and pre-teen girl by CDC measuring BPA urine concentrations. The NTP-CERHR reviewed this and other body burden studies. They conclude that overall the urine monitoring data suggests typical daily intakes of bisphenol A between 0.02 and 0.06 ug BPA per kg bodyweight per day (ug/kg-d) in American adults and children (CERHR 2006).
Our analyses also show that a serving of the food (within each food type tested) with the highest BPA levels result in doses 10 to 20 times higher than those deemed 'typical' by CERHR's review document, and exceed the highest exposures measured in two CDC biomonitoring studies of nearly 500 adults and 7 year-old girls (Calafat 2005; Wolff 2006). These estimates are labeled "high-end food exposures" in the chart below.
People's BPA exposures overlap with doses shown to harm laboratory animals
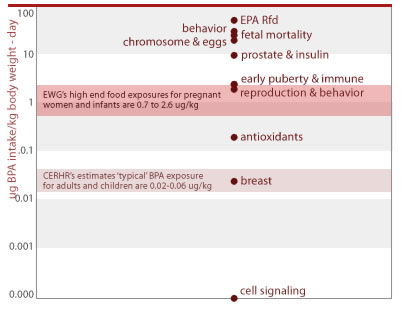
Several important factors can drive people's exposures to BPA even higher than the estimates above, including portion size and differences in infant size and consumption rates.
Larger portions. Our calculations are based on 'official' USDA serving sizes, which underestimate the amounts many Americans eat. For instance, one 15 oz can of chicken soup, considered to be two servings by the government, could easily be eaten in a single meal. The BPA dose for a pregnant women eating this can of soup would be 2.6 ug/kg body weight, and would exceed the doses in the most sensitive studies with absolutely no margin of safety.
Exposure considerations for infants. EWG detected BPA in 2 of 6 concentrated infant formulas. Our calculations suggest that infants consuming concentrated formulas may be among the most highly exposed in the population. Prior to serving concentrated formula to an infant, parents and other caregivers dilute it 50 percent with water. We calculate that a 3-month-old child eating formula with detectable BPA would have ingested 0.76 or 1.2 ug/kg-day from the 2 formulas with detectable BPA in our sample of 6 cans.
A 3-month-old infant of average weight and appetite being nourished exclusively from these formulas would receive concentrations of BPA approaching those in the lowest dose toxicity studies. Since infants typically eat the same brand of formula repeatedly, these harmful exposures could occur daily for their first 6 months of life. Lighter infants or those who drink more than 30 oz per day would have an even more intense exposure. FDA's exposure calculations have used the same daily intake but a 4 kg infant, for an even higher relative dose (Bailey, FDA 1996).
There are few published studies on BPA levels in other formula or other foods eaten by infants. One study by Taiwanese researchers determined the levels of BPA in six brands of canned powdered infant formula. BPA was detected in all samples at concentrations ranging from 45 to 113 parts per billion (ug/kg), much higher than levels detected in the concentrated formula samples tested by EWG (Kuo 2004). The authors calculate a 2.3 ug/kg-d exposure from these formulas alone for a 3-month-old infant (Kuo 2004). These results are greater than EWG's findings for concentrated liquid formula and would need to be confirmed in US formula samples and in a second laboratory. In the meantime there is a critical data gap about the magnitude and safety of infant exposures.
Assessment of canned food exposures versus CDC's measured exposures in 200 women. We conducted analyses to find if the levels of BPA in canned food found in our study could account for a substantial fraction of CDC's measured exposures in the general population, as is expected. These preliminary assessments confirm the dominance of canned food as a BPA exposure source, but also suggest that other sources may be important, particularly in the lower ranges of exposure.
To conduct this analysis, we relied on the government's National Health and Examination Survey (CDC 2002) for data on canned food consumption. This survey, which we maintain in-house in an electronic database, contains one-day, detailed consumption records for 1,929 women of childbearing age (ages 15-44), of whom 1,887 report their body weight. We coupled the canned food consumption data and body weight information from this database with the BPA concentrations from this food survey, averaged by food type.
We assessed three scenarios, one in which we analyzed the one-day exposures for each woman in this database who specifically reported eating food from a can, one for which we include reported soda consumption in addition to canned foods, and a third for which we include potential BPA exposures from foods that can contain canned foods as significant ingredients. For soda and foods that may in part be made from canned ingredients, we may overestimate BPA exposures — the consumption database does not distinguish the fraction of soda contained in cans versus other containers; or the fraction of foods made from canned ingredients. For the purpose of this preliminary calculation, in both these cases we assume that all of the food is contaminated with BPA at the levels we have measured.
We compare the distributions of exposure generated in the final scenario with the exposures indicated by CDC's measurements of BPA in 200 women. We have assumed that CDC's measurements are representative of population-wide exposures for women of childbearing age. These analyses, shown in the chart below, indicate that canned food is a substantial source of BPA exposure. Our analyses under-predict BPA in the low ranges of exposure, suggesting that other sources may be significant in these ranges. Further research in this area should include the use of consumption databases with more comprehensive information on canned food consumption than that provided in government databases. This information is available from commercial sources.
EWG's tests show that canned foods are a significant fraction of people's total BPA exposure
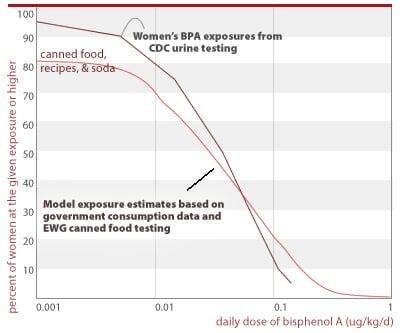
Exposures above reflect the following single-day consumption rates from NHANES: 69% of women report eating canned food or soda (does not include foods with canned ingredients); 40% - 1 serving; 20% - 2 servings; 6.9% - 3 servings; 1.6% - 4 servings; 0.5% - 5+ servings. For canned food consumption only (no soda, no accounting of food made with canned ingredients) consumption rates are: 11.7% - 1 serving; 1.5% - 2 servings; 0.15% - 3 servings.
Chronic exposure estimates show potential for high exposures throughout pregnancy
We conducted analyses to predict average, chronic exposure levels for women who regularly eat canned food. As with our predictions for single-day exposures, we relied on body weight information for women of childbearing age (15-44) from CDC's National Health and Examination Survey. We conducted a Monte Carlo simulation in which women were sequentially assigned BPA concentration and food consumption types and quantities from our underlying databases, for 280 days throughout pregnancy. We calculated each woman's average BPA exposure during pregnancy as the sum of all of her individual, daily BPA exposures divided by the duration of pregnancy. For each woman included in the simulation and for each "food eating" event in her pregnancy, we randomly assigned a BPA concentration from among our canned food test results, and randomly assigned a serving size from among the serving sizes reported in NHANES for the particular food type assumed. For each simulated "eating occasion," we randomly selected the food type consumed using a weighting function that reflected the relative chance each food type would be consumed by a woman of childbearing age on any given day.
EWG analyzed chronic exposures for women who eat either 1, 2, or 3 servings of canned food daily throughout pregnancy. We compared the percent of women in each of these scenarios who would exceed a given dose, against a number of toxic doses measured in lab studies. In each case, we found that significant fractions of women who regularly eat canned food would exceed safe levels of BPA exposures on average throughout pregnancy. Our analysis relies on government canned food consumption data and measured BPA levels from our tests of commonly eaten canned foods. We did not include soda consumption in these analyses.
Many women who eat canned food are exposed to unsafe levels of BPA
All women eating 1, 2, or 3 servings of canned food per day, respectively, would get an average BPA dose above that linked to breast cancer and damage of the female reproductive system (0.025 ug/kg/d).
0.025 ug/kg/d
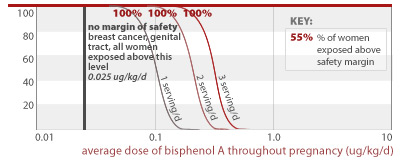
54% and 95% of women eating 2 or 3 servings of canned food per day, respectively, would get an average BPA dose less than a factor of 10 away from doses linked to prostate damage and aggression in addition to effects listed for higher doses above (2.0 ug/kg/d). All women eating 1, 2, or 3 servings of canned food daily are exposed to BPA doses within a factor of 100 from this toxic dose.
2.0 ug/kg/d
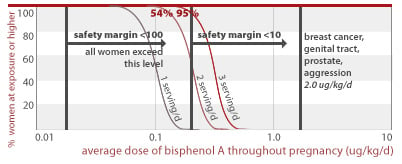
24% and 84% of women eating 1 to 3 servings of canned food per day throughout pregnancy would get an average BPA dose less than a factor of 10 away from doses linked to early puberty and diabetes (2.4 ug/kg/d) in addition to effects listed for higher doses above. All women eating 1, 2, or 3 servings of canned food daily are exposed to BPA doses within a factor of 100 from this toxic dose.
2.4 ug/kg/d
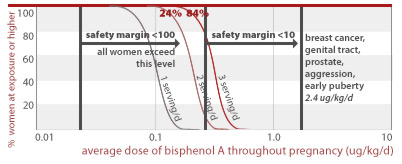
53%, 99.6%, and 100% of women eating 1, 2, or 3 servings of canned food per day throughout pregnancy, respectively, would get an average BPA dose less than a factor of 100 away from doses linked to prostate damage and diabetes in addition to effects listed for higher doses above (10 ug/kg/d).
10 ug/kg/d
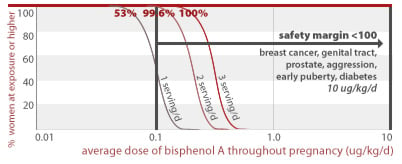
2.6% of women eating 2 servings of canned food per day throughout pregnancy would get an average BPA dose less than a factor of 100 away from a dose linked to structural damage of the brain (30 ug/kg/d). 55% of women eating 3 servings of canned food per day throughout pregnancy would also exceed this margin of safety.
30 ug/kg/d
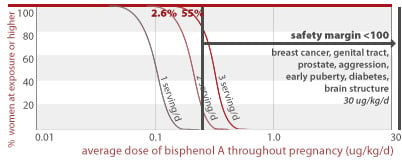
BPA and human diseases on the rise
A recent study from the Centers for Disease Control tested a demographically diverse group of almost 400 Americans for evidence of exposure to BPA and found that 95% of study participants had the chemical in their urine (Calafate 2005; Wolff 2007). BPA has been linked to a variety of health outcomes which are prevalent and in many cases increasing in the United States and responsible for a major toll on our collective health. These include breast and prostate cancer, and infertility (Maffini 2006).
BPA's toxic effects in lab animals are on the rise and common in people
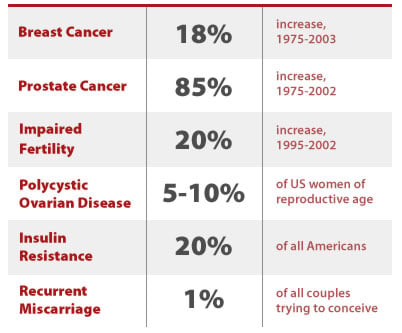
Female reproductive effects linked to BPA exposure
There are few published studies on the effects of exposure to BPA in humans. Japanese scientists found that women with polycystic ovarian syndrome had higher serum levels of BPA relative to women with normal ovarian function, and that there were positive correlations between BPA concentrations and androgen levels (Takeuchi et al. 2006). Polycystic ovarian syndrome is the most common form of female infertility in the U.S., affecting 5 to 10 percent of American women.
Another study found an inverse relationship between BPA concentrations and the presence of complex endometrial hyperplasia; the authors suggested that this surprising finding pointed to a more complex relationship between BPA exposure and estrogen dependent diseases than previously thought. (Hiroi, Tsutsumi et al. 2004) Lastly, a study of women with a history of recurrent miscarriages found they had higher serum BPA when compared with women with normal pregnancies, leading the authors of the study to conclude that "exposure to bisphenol A is associated with recurrent miscarriage" (Sugiura-Ogasawara et al. 2005). Recurrent miscarriages affect one percent of American couples trying to conceive (Rai 2006).
Male reproductive effects linked to BPA exposure
Men with occupational exposure to epoxy resins were found to have decreased secretion of follicle stimulating hormone when compared with men without occupational exposure to epoxy resins (Hanaoka et al. 2002). Follicle stimulating hormone is critical to sperm formation; diminished secretion of this hormone in men can result in reduced sperm concentration and infertility.
American diseases trends show increase in impacts related to BPA in lab animals
Exposure to Bisphenol A is widespread in the United States, and it has been linked to a number of adverse health effects in animal studies, including breast and prostate cancers, impaired fertility, and insulin resistance (Maffini 2006). While a few studies link BPA exposure to altered reproductive system function in women and men, but these human studies are costly and complicated to perform. Thus animal studies will always provide an indication of potential impacts to human health. The health impacts of BPA exposure in animals bear special consideration in light of disease trends in the American population.
The US is noted to have one of the highest incidence rates for breast and prostate cancers in the world; lifetime risk for these cancers has steadily risen over the last two decades. Cancer rates now reflect a 1 in 8 lifetime risk of breast cancer for women, and a 1 in 6 risk of prostate cancer for men (SEER 2006).
Invasive female breast cancer increased an average of 1.5 percent per year between 1973 and 1996, for a total increase of 25.3 percent. Among those 65 and younger, breast cancer incidence rose 1.2 percent per year, corresponding to a doubling every two generations (58 years).
Prostate cancer rates increased by 85 percent between 1975 and 2002 (SEER 2006). Part of this increase can be explained by better detection, but increased incidence has also been accompanied by an increase in mortality - which better detection cannot explain. Prostate cancer is now the most common cancer among U.S. men, and the second most lethal, killing an estimated 27,000 men in the year 2007 alone (SEER 2007).
Infertility rates are notoriously difficult to track, but researchers estimate a 20 percent increase in the last decade, with an estimated 7.3 million American couples currently facing infertility (Barrett 2006). As mentioned above, polycystic ovarian syndrome affects 5 to 10 percent of women of childbearing age, and is a leading cause of infertility (Jakubowicz 2002). In addition, reduced secretion of follicle stimulating hormone can impair fertility in men. Epidemiological studies link BPA to polycystic ovarian syndrome in women, and reduced secretion of follicle stimulating hormone in men (Takeuchi et al. 2006; Hanaoka et al. 2002).
Insulin resistance has also increased in incidence and is a major predictor for type II diabetes; interestingly, women with polycystic ovarian syndrome also often develop insulin resistance as well (AHA 2007). It is estimated that 30 million people in the US may have insulin resistance.
A recent study from Europe has also found a link between exposure to low doses of BPA and insulin resistance. In this study, adult mice that were exposed to low doses of BPA — 10 ug/kg/day for four days (Alonso-Magdalena, Morimoto et al. 2006). The exposed animals were found to have sustained increases in serum insulin levels after just two days of exposure and impaired glucose tolerance after four days. Increased insulin levels are associated with Type 2 diabetes.
A relationship between diabetes and environmental contaminants is beginning to emerge in the scientific literature. Dioxin exposure was first discovered as a contributing factor to diabetes among military personnel who worked with dioxin-containing Agent Orange during the Vietnam War. A link to six other persistent toxins was recently reported in a study of 2000 North Carolinans (Lee, Lee et al. 2006, as reported in OSF 2006). While obesity is often thought of as the major risk factor for diabetes, it was not a significant contributor to disease in those participants with the lowest-level contaminant exposure. Further research will clarify the contribution of environmental chemicals to the diabetes epidemic. In the meantime, the possibility that BPA plays role in provoking insulin resistance in humans bears more attention.
Causal relationships between environmental exposures and health effects are often difficult to establish because of many factors, including non-standardized diagnosis of diseases, difficulties ascertaining exposure to ubiquitous environmental contaminants, and inadequate statistical power to address other contributing factors. Endocrine disruptors, such as BPA, may very well play a part in the etiology of classic reproductive disorders and cancers, as well as diseases not often linked to hormonal activity — immune system conditions, learning and behavioral disorders, diabetes, and even obesity. If BPA does indeed contribute to any of these epidemic disorders, the potential ramifications for public health are far-reaching. Toxicity studies involving rodent exposure to BPA should not be considered in a void, but rather should be framed within the current context of clinical concerns for the U.S. population.
Companies reduced BPA exposures in Japan
Food manufacturers in other countries appear to be taking voluntary measures to reduce BPA contamination in food. US manufacturers should do the same without waiting for the government to set stronger safety standards for this toxic chemical.
Japanese scientists, government and industry have all taken a notable interest in BPA exposure and reduction strategies. Due to consumer concern about the toxic effects of BPA, Japanese industries voluntarily reduced the use of BPA dramatically between 1998 and 2003.
In 1998 BPA concentrations ranging from 0.6-1 ug/L were detected in 12 of 20 canned drinks in Japan. According to the Japanese government, voluntary efforts by can manufactures reduced the migration level a goal of <5 ug/l. To do so they changed the inner surface of the cans from EXR coating to PET film lamination, or they used a EXR paint with much less BPA migration into food. Due to these BPA reduction and inactivation measures, the assessors noted that virtually no BPA is detected in canned foods and beverages now. Also in Japan, polycarbonate tableware in school lunches were largely replaced with the safer alternatives of polypropylene or melamine, ABS resin, polyethylene naphthalate and stainless steel (RCCRM 2005).
Japanese efforts to reduce human exposure to BPA appear to have paid off with diminished BPA exposure. Japanese risk assessors estimate that the reduced intake of BPA from the cans and tableware changes was 0.3 to 0.5 ug/kg/day per child. But people consuming the most drinks would have an estimated reduction of 0.6 ug/kg/d from drinks alone (Junko 2005).
A group of researchers studying BPA exposure for college students noted a greater than 50 percent decline in BPA measurements in groups of college students examined before and after canned foods and tableware were redesigned. Before the intervention, BPA detections in blood were strongly correlated with the frequency that students drank warm beverages, namely coffee and tea which are commonly contained in cans in Japan. After the redesigned cans were introduced, the frequency of consuming canned drinks had no relationship to BPA measurements, which is what one would expect if BPA levels had been reduced (Matsumoto 2003).
According to the United Kingdom Food Standards Agency the food industry in the U.K. may also be taking voluntary steps to minimize BPA leaching from cans: "Industry is taking action to reduce levels of bisphenol A in canned food to as low as possible and is investigating alternatives to this substance" (UKFSA 2001).
Since alternatives to BPA appear to be both available and feasible, U.S. manufacturers should take action now to reduce their customer's exposures to this toxic chemical.
Tables
EWG test results — BPA is common contaminant in name-brand canned foods heavily consumed by women and infants
| Canned Foods | Number of brands tested | Number of cans tested | Foods tested |
BPA % detect | Average BPA level* and range (ppb) |
|---|---|---|---|---|---|
| All foods | 30 | 97 | 57% | 7.9 (ND - 385) | |
| Beans | 3 | 6 | baked beans | 83% | 9.7 (ND - 38) |
| Fruit | 6 | 17 | mixed fruit, cranberry sauce, peaches, pears, pineapple | 35% | 2.3 (ND - 27) |
| Infant formula | 2 | 6 | concentrated infant soy and milk-based formula | 33% | 2.4 (ND - 17) |
| Meal replacement | 2 | 5 | liquid meal replacements | 40% | 4.2 (ND - 66) |
| Milk products | 3 | evaporated milk | 66% | 3.5 (ND - 9) | |
| Pasta | 2 | 6 | ravioli, spaghetti | 100% | 63.5 (16 - 247) |
| Soda | 2 | 12 | cola, diet cola | 42% | 1.7 (ND - 8) |
| Soup | 5 | 19 | beef stew, chicken noodle, chicken rice, chicken vegetable, tomato, vegetable | 89% | 57.6 (ND - 385) |
| Tuna | 2 | 6 | chunk lite, solid white | 50% | 9.6 (ND - 108) |
| Vegetable | 8 | 17 | corn, green beans, mixed vegetables, peas, tomatoes | 41% | 7.8 (ND - 330) |
BPA concentrations are expressed in parts per billion (ppb) by weight (micrograms of BPA per kilogram of food).
* Average is the geometric mean. Non-detects considered to be 1/2 the detection limit (1 ppb) for purposes of this calculation.
BPA levels in individual cans - from EWG's test program of 97 cans of 30 name-brand foods
| Type of canned food | Specific food type | State of purchase | Bisphenol A (ppb)# | Serving size (oz) + | Average BPA exposure from single serving (ug/kg-d)* |
|---|---|---|---|---|---|
| Beans | baked beans | GA | <2 | 4.1 | ND |
| Beans | baked beans | GA | 37.7 | 4.6 | 0.08 |
| Beans | baked beans | CA | 27.1 | 4.7 | 0.06 |
| Beans | baked beans | CT | 27 | 4.0 | 0.05 |
| Beans | baked beans | CT | 6.34 | 4.1 | 0.01 |
| Beans | baked beans | CA | 4.83 | 4.1 | 0.01 |
| Fruit | cranberry sauce | CA | <2 | 2.7 | ND |
| Fruit | cranberry sauce | CT | <2 | 2.7 | ND |
| Fruit | cranberry sauce | GA | <2 | 2.7 | ND |
| Fruit | mixed fruit | CA | <2 | 4.3 | ND |
| Fruit | mixed fruit | CA | <2 | 4.1 | ND |
| Fruit | mixed fruit | CT | <2 | 4.1 | ND |
| Fruit | mixed fruit | GA | <2 | 4.3 | ND |
| Fruit | mixed fruit | GA | <2 | 4.4 | ND |
| Fruit | mixed fruit | CT | 10.6 | 4.4 | 0.02 |
| Fruit | peaches | GA | <2 | 4.4 | ND |
| Fruit | peaches | CT | 7.43 | 4.2 | 0.01 |
| Fruit | pears | CT | <2 | 4.4 | ND |
| Fruit | pears | CA | 15.6 | 4.4 | 0.03 |
| Fruit | pears | GA | 14 | 4.3 | 0.03 |
| Fruit | pineapple | GA | <2 | 4.4 | ND |
| Fruit | pineapple | CT | 26.9 | 4.4 | 0.06 |
| Fruit | pineapple | CA | 2.2 | 4.0 | 0.00 |
| Infant formula | milk formula with iron | CT | <2 | 30.0 | ND |
| Infant formula | milk formula with iron | GA | <2 | 30.0 | ND |
| Infant formula | milk formula with iron | CA | 17.1 | 30.0 | 1.20 |
| Infant formula | milk formula with iron | GA | 10.9 | 30.0 | 0.76 |
| Infant formula | soy formula with iron | CA | <2 | 30.0 | ND |
| Infant formula | soy formula with iron | CT | <2 | 30.0 | ND |
| Meal replacement | chocolate shake | CA | <2 | 11.0 | ND |
| Meal replacement | chocolate shake | CA | <2 | 11.0 | ND |
| Meal replacement | chocolate shake | CT | <2 | 11.0 | ND |
| Meal replacement | chocolate shake | GA | 65.5 | 11.0 | 0.34 |
| Meal replacement | vanilla shake | GA | 19.3 | 11.0 | 0.10 |
| Other | evaporated milk | CT | <2 | 1.0 | ND |
| Other | evaporated milk | GA | 9 | 1.0 | 0.00 |
| Other | evaporated milk | CA | 4.83 | 1.0 | 0.00 |
| Pasta | ravioli | CA | 247 | 7.5 | 0.87 |
| Pasta | ravioli | GA | 220 | 7.5 | 0.78 |
| Pasta | ravioli | CT | 16.2 | 7.5 | 0.06 |
| Pasta | spaghetti | CA | 52.9 | 7.5 | 0.19 |
| Pasta | spaghetti | GA | 38.1 | 7.5 | 0.13 |
| Pasta | spaghetti | CT | 37.1 | 7.4 | 0.13 |
| Soda | cola | CA | <2 | 12.5 | ND |
| Soda | cola | CT | <2 | 8.4 | ND |
| Soda | cola | CT | <2 | 8.4 | ND |
| Soda | cola | CA | 4.19 | 12.5 | 0.02 |
| Soda | cola | GA | 3.35 | 12.5 | 0.02 |
| Soda | cola | GA | 2.41 | 8.4 | 0.01 |
| Soda | diet cola | CA | <2 | 12.5 | ND |
| Soda | diet cola | CT | <2 | 8.4 | ND |
| Soda | diet cola | CT | <2 | 8.4 | ND |
| Soda | diet cola | GA | <2 | 8.4 | ND |
| Soda | diet cola | CA | 8.21 | 12.5 | 0.05 |
| Soda | diet cola | GA | 2.74 | 12.5 | 0.02 |
| Soup | beef stew | CT | 26.9 | 9.4 | 0.12 |
| Soup | beef stew | CA | 19 | 9.4 | 0.08 |
| Soup | chicken broth | CT | 8.64 | 7.0 | 0.03 |
| Soup | chicken noodle soup | GA | <2 | 4.3 | ND |
| Soup | chicken noodle soup | CT | 385 | 7.2 | 1.32 |
| Soup | chicken noodle soup | CT | 184 | 4.2 | 0.37 |
| Soup | chicken noodle soup | CA | 83.3 | 4.3 | 0.17 |
| Soup | chicken rice soup | GA | 121 | 4.2 | 0.24 |
| Soup | chicken rice soup | CT | 104.4 | 4.2 | 0.21 |
| Soup | chicken rice soup | CA | 103 | 4.2 | 0.20 |
| Soup | chicken vegetable soup | CA | 122 | 9.5 | 0.55 |
| Soup | chicken vegetable soup | CT | 49.1 | 9.5 | 0.22 |
| Soup | noodle soup | CA | 191 | 4.4 | 0.40 |
| Soup | noodle soup | GA | 99.3 | 4.2 | 0.20 |
| Soup | other soup | CT | <15 | 7.1 | ND |
| Soup | tomato soup | CA | 176 | 4.3 | 0.36 |
| Soup | tomato soup | CT | 88.5 | 4.3 | 0.18 |
| Soup | tomato soup | GA | 78.2 | 4.3 | 0.16 |
| Soup | vegetable soup | CA | 79.6 | 9.2 | 0.35 |
| Tuna | chunk lite | CA | <2 | 2.4 | ND |
| Tuna | chunk lite | CT | 108 | 2.4 | 0.12 |
| Tuna | chunk lite | CA | 89.8 | 2.4 | 0.10 |
| Tuna | chunk lite | GA | 80 | 2.4 | 0.09 |
| Tuna | chunk white | GA | <2 | 2.4 | ND |
| Tuna | solid white | CT | <2 | 2.4 | ND |
| Vegetable | corn | CT | <2 | 4.4 | ND |
| Vegetable | corn | GA | <2 | 2.7 | ND |
| Vegetable | green beans | CA | <2 | 4.1 | ND |
| Vegetable | green beans | GA | 284 | 4.1 | 0.56 |
| Vegetable | green beans | CT | 209 | 4.1 | 0.41 |
| Vegetable | mixed vegetables | CA | <2 | 4.1 | ND |
| Vegetable | mixed vegetables | CT | <2 | 4.1 | ND |
| Vegetable | mixed vegetables | CT | <2 | 4.3 | ND |
| Vegetable | mixed vegetables | GA | <15 | 4.3 | ND |
| Vegetable | mixed vegetables | GA | 330 | 4.1 | 0.65 |
| Vegetable | mixed vegetables | CA | 225 | 4.3 | 0.46 |
| Vegetable | peas | CT | <2 | 4.3 | ND |
| Vegetable | peas | CA | 203.5 | 4.3 | 0.41 |
| Vegetable | peas | GA | 22.7 | 4.3 | 0.05 |
| Vegetable | tomatoes | CA | <2 | 2.2 | ND |
| Vegetable | tomatoes | CT | <2 | 4.1 | ND |
| Vegetable | tomatoes | GA | 8.94 | 2.2 | 0.01 |
Source: Chemical analyses of 97 canned foods by Southern Testing and Research Division of Microbac Laboratories, Inc., North Carolina.
# BPA concentrations are expressed in parts per billion (ppb) by weight (micrograms of BPA per kilogram of food)
+ Serving size as noted on can label.
* BPA exposure is expressed in ug/kg-d, or micrograms of BPA per kilogram of body weight per day. For comparison, numerous animal studies show toxic effects at 2 ug/kg/d and lower.
EWG estimated the BPA dose from single serving of food using the following assumptions: BPA calculations reflect a single adult serving, using label serving size and body weight of 60 kg (132 lbs); exposures for concentrated infant formula is calculated for exclusively formula-fed infant using average 3-month-old body weight (6 kg/13 lbs) and average daily formula ingestion (840 g/30 oz); formula is assumed diluted with water free of BPA.
Summary of BPA measurements in canned food from 9 previous studies
| Food type | Number of studies | Location | Total number of cans tested | Percent of cans with BPA detected | BPA range, ppb (ug/kg) | EWG study: BPA range, ppb (ug/kg) | Ref- erences |
|---|---|---|---|---|---|---|---|
| Beverages | 1 | Austria | 7 | 0% | <0.9 - 3.4 | 2.4 - 8.2 | [2] |
| Canned meat+ | 3 | New Zealand, UK | 10 | ~75% | 8.6 - 89 | NA | [5, 6, 9] |
| Fruit | 2 | Austria, UK | 6 | >80% | 5 - 38 | 2.2 - 27 | [2, 5] |
| Fruit & vegetables | 1 | New Zealand | 38 | unavailable | <20 - 24 | NA | [9] |
| Infant food | 2 | New Zealand, UK | 10 | 30% | <10 - 77 | NA | [5, 9] |
| Infant formula | 3 | US, UK, Taiwan | 24 | 80% | <0.002 - 113 | 10.9 - 17.1 | [1, 5, 7] |
| Pasta | 3 | New Zealand, UK | 10 | >50% | <7 - 130 | 16.2 - 247 | [5, 6, 9] |
| Soup | 3 | New Zealand, UK | 15 | unavailable | <2 - 39 | 8.6 - 385 | [5, 6, 9] |
| Tuna | 4 | New Zealand, UK, Mexico, Austria | 16 | 75% | <7 - 109 | 80 - 108 | [5, 8, 9, 10] |
| Vegetables | 5 | Austria, UK, Spain, US | 34 | >80% | 4 - 76 | 8.9 - 330 | [2, 3, 4, 5, 6] |
+ Does not include tuna
References
U.S.: [1] Biles, J. E., McNeal, T. P. and Begley, T. H. Determination of bisphenol A migrating from epoxy can coatings to infant formula liquid concentrates. J Agric Food Chem 1997; 45: 4697-4700.
Austria: [2] Braunrath, R., Podlipna, D., Padlesak, S. and Cichna-Markl, M. Determination of bisphenol A in canned foods by immunoaffinity chromatography, HPLC, and fluorescence detection. J Agric Food Chem 2005; 53: 8911-7.
Spain: [3] Brotons, J. A., Olea-Serrano, M. F., Villalobos, M., Pedraza, V. and Olea, N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect 1995; 103: 608-12.
U.S.: [4] FDA. Cumulative Exposure Estimated for Bisphenol A (BPA), Individually for Adults and Infants from Its Use in Epoxy-Based Can Coatings and Polycarbonate (PC) Articles, verbal request of 10-23-95, memorandum to G. Diachenki, Ph.D, Division of Product Manufacture and Use, HGS-245, from Allan B. Bailey, Ph.D., Chemistry Review Branch, HFS-245. Department of Health and Human Services, Food and Drug Administration. Food and Drug Administration; 1996.
U.K.: [5] Goodson, A., Robin, H., Summerfield, W. and Cooper, I. Migration of bisphenol A from can coatings--effects of damage, storage conditions and heating. Food Addit Contam 2004; 21: 1015-26.
U.K.: [6] Goodson, A., Summerfield, W. and Cooper, I. Survey of bisphenol A and bisphenol F in canned foods. Food Addit Contam 2002; 19: 796-802.
Taiwan: [7] Kuo, H.-W. and Ding, W.-H. Trace determination of bisphenol A and phytoestrogens in infant formula powders by gas chromatography-mass spectometry. J Chromatogr A 2004; 1027: 67-74.
Mexico: [8] Munguía-López , E. M., Gerardo-Lugo, S., Peralta, E., Bolumen, S. and Soto-Valdez, H. Migration of bisphenol A (BPA) from can coatings into a fatty-food simulant and tuna fish. Food Addit Contam 2005; 22: 892-8
New Zealand: [9] Thomson, B. M. and Grounds, P. R. Bisphenol A in canned foods in New Zealand: an exposure assessment. Food Addit Contam 2005; 22: 65-72.
BPA in a single serving of many foods tested would exceed a minimal margin of safety from the low dose effects of oral exposure (2.0 ug/kg-d)+
| Food Type | Number of cans tested | Percent of cans with single-serving dose within margin of 5 from harmful level | Percent of cans with single-serving dose within margin of 10 from harmful dose | Percent of cans with single-serving dose within margin of 100 from harmful dose | High-end daily intake for consumer* (ug/kg-d) |
|---|---|---|---|---|---|
| Pasta | 6 | 33% | 33% | 100% | 0.87 |
| Infant formula# | 6 | 33% | 33% | 33% | 1.20 |
| Vegetable | 17 | 29% | 29% | 35% | 0.65 |
| Soup | 19 | 11% | 53% | 89% | 1.32 |
| Meal replacement | 5 | 0% | 20% | 40% | 0.34 |
| Tuna | 6 | 0% | 0% | 50% | 0.12 |
| Beans | 6 | 0% | 0% | 50% | 0.08 |
| Fruit | 17 | 0% | 0% | 24% | 0.06 |
| Soda | 12 | 0% | 0% | 17% | 0.05 |
| Milk products | 3 | 0% | 0% | 0% | 0.004 |
| All foods | 97 | 11% | 21% | 46% | NA |
*Calculated for a single serving of the can with the maximum BPA detection for that food type
# Serving = average daily intake for 3 month-old infant exclusively formula fed
+ Nagel et al. 1997
Many studies confirm BPA's low-dose toxicity across a diverse range of toxic effects
| Daily BPA exposure (ug/kg body weight-day) | CERHR conclusion* | Toxic effect | Study details | Reference | % cans tested by EWG with single-serving BPA levels within a margin of 10 from harmful dose |
|---|---|---|---|---|---|
| 0.0001 | not included | alterations in cell signalling pathways on the cell surface that control calcium eflux in cells | in-vitro study which compared activity of BPA and other hormone disruptors | Wozniak 2005 | 56.7 (all cans with detected BPA) |
| 0.025 | "very useful" | persistent changes to breast tissue, predisposes cells to hormones and carcinogens | fetal exposure, osmotic pumps, changes noted a 6 months of age | Muñoz-de-Toro 2005 | 55.7 |
| 0.025 | "useful and shows tissue effects at extremely low dose levels" | permanent changes to genital tract | fetal exposure, osmotic pumps | Markey 2005 | 55.7 |
| 0.2 | utility "limited" | decrease antioxidant enzymes | adult exposure, oral | Chitra 2003 | 47.4 |
| 0.25 | utility "to be added" | altered growth, cell size and lumen formation in mammary epithelium of mouse fetuses. | exposure during pregnancy w/osmotic pumps | Vandenberg 2007 | 45.4 |
| 2 | "useful" | increased prostate weight 30% | fetal exposure, oral route | Nagel 1997 | 20.6 |
| 2 | "moderately useful" | increased aggression at 8 weeks of life | fetal exposure, oral route | Kawai 2003 | 20.6 |
| 2.4 | "useful", but non-traditional endpoint | Decreased time from vaginal opening to first estrus, possibly earlier puberty | fetal exposure, oral route | Howdeshell 1999 | 17.5 |
| 2.4 | "useful" | lower bodyweight, increase of anogenital distance in both genders, signs of early puberty and longer estrus. | fetal exposure, oral route | Honma 2002 | 17.5 |
| 2.4 | "adequate" | decline in testicular testosterone | fetal and neonatal exposure, gavage | Akingbemi 2004 | 17.5 |
| 2.5 | utility "to be added" | breast cells predisposed to cancer | fetal exposure, osmotic pumps | Murray 2007 | 16.5 |
| 2.5 | not included | immune system impacts | oral exposure | Sawai 2003 | 16.5 |
| 10 | utility "very useful" | prostate cells more sensitive to hormones and cancer | infant oral exposure, 3 day duration | Ho 2006 | 2.1 |
| 10 | utility "very useful" | prostate cells more sensitive to hormones and cancer | fetal exposure, oral route, short duration | Timms 2005 | 2.1 |
| 10 | not included | insulin resistance develops in 2 days, chronic hyperinsulinemia at day 4 | subcutaneous injection, short duration exposure | Alonso-Magdalena 2006 | 2.1 |
| 10 | "very useful" | decreased maternal behaviors | fetal and neonatal exposure, oral route | Palanza 2002 | 2.1 |
| 20 | not included | damage to eggs and chromosomes | fetal exposure, osmotic pumps | Hunt 2003 | 0 |
| 20 | not included | damage to eggs | fetal exposure, osmotic pumps | Susiarjo 2007 | 0 |
| 20 | not included | brain effects - disrupted neocortical development by accelerating neuronal differentiation and migration | single injection | Nakamura 2006 | 0 |
| 30 | "...adequate for the evaluation process and gives cause for concern" | reversed the normal sex differences in brain structure and behavior | oral during gestation and lactation | Kubo 2003 | 0 |
| 30 | "suitable" | hyperactivity | oral | Ishido 2004 | 0 |
| 50 | EPA RfD | EPA's 'safe exposure level, based on outdated, high dose studies and a 1000-fold margin of safety | EPA 1998 | 0 |
*CERHR conclusion refers to the Center for Evaluation of Risks to Human Reproduction expert panel assessment of the utility of the study in the panel's review of BPA risks to human reproduction (CERHR 2006).
Statistics on percent cans with single servings that would yield human dose within a margin of 10 of the toxic dose are generated with the following assumptions: BPA calculations reflect a single adult serving, using label serving size and body weight of 60 kg (132 lbs); exposures for concentrated infant formula is calculated for exclusively formula-fed infant using average 3-month-old body weight (6 kg/13 lbs) and average daily formula ingestion (840 g/30 oz); formula is assumed diluted with water free of BPA.
Consumer tips to avoid BPA exposure
Although completely eliminating exposure to BPA may not be possible, there are steps you can take to reduce your family's exposure to this chemical.
Infant formula: All U.S. manufacturers use BPA-based lining on the metal portions of the formula containers. Tests of liquid formulas by FDA and EWG show that BPA leaches into the formula from all brands tested. Enfamil formula appears to have the highest concentrations of the 20 tests. EWG is concerned about BPA exposures for babies fed liquid formula. Choose powdered formula which may not have BPA in packaging and which is more diluted with water. If your baby needs liquid formula look for types sold in plastic or glass containers.
Our testing of canned foods found that BPA leaches from the liner into the food itself. Sensitive groups such as kids and pregnant women should limit canned food consumption. Beverages appear to contain less BPA residues, while canned pasta and soups contain the highest levels. Rinsing canned fruit or vegetables with water prior to heating and serving could lessen BPA ingestion.
Certain plastics called polycarbonates leach low levels of BPA into food or liquids. Leaching from plastic baby bottles and food containers appears to happen at a much lower level than found in canned foods and baby formula. Nevertheless it is good to take simple precautions.
BPA is found in polycarbonate plastic food containers often marked on the bottom with the letters "PC" recycling label #7. Not all #7 labeled products are polycarbonate but this is a reasonable guideline for a category of plastics to avoid. Polycarbonate plastics are rigid and transparent and used for sippy cups, baby bottles, food storage, and water bottles. Some polycarbonate water bottles are marketed as 'non-leaching' for minimizing plastic taste or odor, however there is still a possibility that trace amounts of BPA will migrate from these containers, particularly if used to heat liquids.
Safer products and uses: When possible it is best to avoid #7 plastics, especially for children's food. Plastics with the recycling labels #1, #2 and #4 on the bottom are safer choices and do not contain BPA. Find baby bottles in glass versions, or those made from the safer plastics including polyamine, polypropylene and polyethylene. Soft or cloudy-colored plastic does not contain BPA. Bottles used to pump and store expressed breast milk by the brand Medela are also labeled BPA-free.
Some metal water bottles are lined with a plastic coating that contains BPA. Look for stainless steel bottles that do not have a plastic liner.
We recommend avoiding use of plastic containers to heat food in microwaves. Ceramic, glass, and other microwaveable dishware are good alternatives. Avoid using old and scratched plastic bottles.






